Displacement Reactions - Definition, Types, Applications, FAQs
What happens when a more reactive element comes in contact with a compound containing a less reactive element? Can one element actually replace another from its compound? You will find these answer by studing displacement reaction. A chemical reaction in which a more reactive element displaces a less reactive element from its compound.
This Story also Contains
- Displacement Reaction
- Types of Displacement Reaction
- Applications of Displacement Reaction:
- Displacement Reaction Examples
- Some Solved Examples
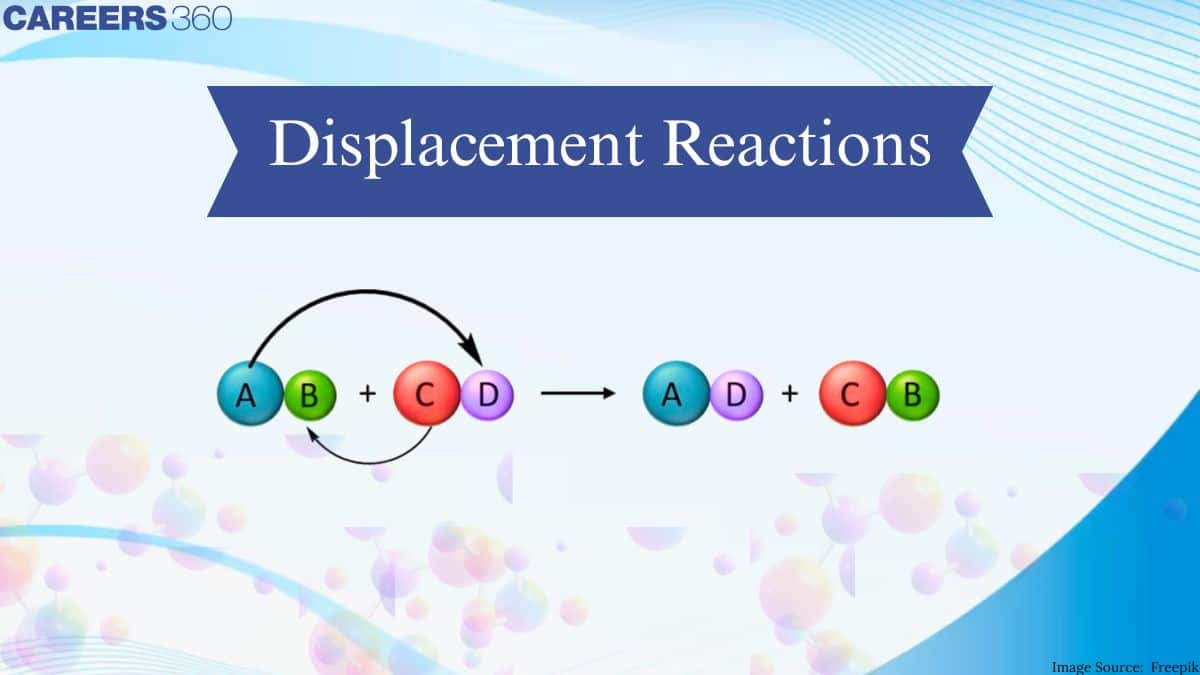
Displacement Reactions are extremely important chemistry processes. They are employed in a variety of fields in a variety of ways. For example, we employ electroplating, which is based on displacement reaction, to keep iron objects from rusting. The term "displacement reaction" refers to a reaction in which a portion of one reactant is displaced by another reactant. It's also known as a substitution reaction. As a result of the replacement of one reactant ion by another reactant ion.
Example: $2 \mathrm{~K}+\mathrm{MgCl}_2 \rightarrow 2 \mathrm{KCl}+\mathrm{Mg}$
This example shows the displacement of magnesium from magnesium chloride by potassium which is more reactive than magnesium.
Also read -
Displacement Reaction
Single Displacement Reaction
Single displacement reactions are those in which one element replace another element from its compound. Single replacement reactions are another name for this type of reaction.
$A+B-C \rightarrow A-C+B$
Example: $\mathrm{Zn}+\mathrm{CuSO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{Cu}$
This is a single displacement reaction in which zinc is more reactive than copper, thus zinc displaces the copper from copper sulfate and forms zinc sulfate and copper.
Double Displacement Reaction
Double displacement reaction definition: Double displacement reaction are those in which two chemicals react by exchanging ions to produce two new molecules. Positive ions exchange negative ion partners in double replacement processes. Ionic chemicals dissolved in water undergo a lot of double displacement processes. The general equation represents a twofold replacement reaction.
$\mathrm{AB}+\mathrm{CD} \rightarrow \mathrm{AC}+\mathrm{BD}$
Atoms from two distinct compounds swap positions in a double displacement process. Two chemicals serve as reactants, while two compounds serve as products. Consider the following scenario: $\mathrm{Fe}_2 \mathrm{O}_3+6 \mathrm{HCl} \rightarrow 2 \mathrm{FeCl}_3+3 \mathrm{H}_2 \mathrm{O}$
Types of Displacement Reaction
Neutralisation Reaction: The acid-base process produces salt (which is naturally neutral) and water. For example, sodium chloride and water are generated when an aqueous solution of hydrochloric acid is mixed with an aqueous solution of sodium hydroxide.
$\mathrm{NaCl}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{NaOH}+\mathrm{HCl}$
Precipitation reaction: The precipitate is generated in a solution during chemical reaction in this reaction. When silver nitrate and sodium chloride are mixed in water, a white curdy silver chloride precipitate is created.
$\mathrm{AgNO}_3+\mathrm{NaCl} \rightarrow \mathrm{AgCl}_2+\mathrm{NaNO}_3$
Applications of Displacement Reaction:
Welding with thermite: The reaction of aluminium and ferrous oxide to produce molten iron, which is used to weld rail rails.
$2 \mathrm{Al}+\mathrm{Fe}_2 \mathrm{O}_3 \rightarrow \mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{Fe}$
Aluminum is placed above the iron in the reactive series, thus aluminum is more reactive than iron which displaces iron from iron oxide to form aluminum oxide and iron.
Blast furnace: The iron is removed from its ore Fe2O3, which is used to make steel, in a blast furnace. Carbon replaces Ferrous in the process, resulting in carbon dioxide. The reaction is:
$3 \mathrm{C}+2 \mathrm{Fe}_2 \mathrm{O}_3 \rightarrow 3 \mathrm{CO}_2+4 \mathrm{Fe}$
Carbon displaces the iron from the iron oxide forming carbon dioxide and iron.
Metal extraction: Metals are formed by heating metal combinations in the presence of carbon. When a Cr2O3 combination is heated, chromium is produced. The reaction will be:
$3 \mathrm{C}+2 \mathrm{Cr}_2 \mathrm{O}_3 \rightarrow 3 \mathrm{CO}_2+4 \mathrm{Cr}$
Displacement Reaction Examples
Single displacement reaction examples –
-
Displacement of copper from copper sulphate –
$\mathrm{Zn}+\mathrm{CuSO}_4 \rightarrow \mathrm{ZnSO}_4+\mathrm{Cu}$
-
Displacement of silver from silver nitrate –
$\mathrm{Cu}+2 \mathrm{AgNO}_3 \rightarrow \mathrm{CuNO}_3+2 \mathrm{Ag}$
-
Displacement of copper from copper sulphate –
$\mathrm{Fe}+\mathrm{CuSO}_4 \rightarrow \mathrm{Cu}+\mathrm{FeSO}_4$
-
Displacement of copper from copper chloride –
$\mathrm{Pb}+\mathrm{CuCl}_2 \rightarrow \mathrm{Cu}+\mathrm{PbCl}_2$
-
Displacement of sodium from sodium bromide –
$\mathrm{Cl}_2+2 \mathrm{NaBr} \rightarrow 2 \mathrm{NaCl}+\mathrm{Br}_2$
Double displacement reaction examples -
-
Displacement of aluminum from aluminum trichloride and potassium from potassium chloride –
$\mathrm{KNO}_3+\mathrm{AlCl}_3 \rightarrow \mathrm{Al}\left(\mathrm{NO}_3\right)_3+\mathrm{KCl}$
-
Displacement of lead from lead nitrate and potassium from potassium iodide –
$\mathrm{Pb}\left(\mathrm{NO}_3\right)_2+2 \mathrm{KI} \rightarrow 2 \mathrm{KNO}_3+\mathrm{PbI}_2$
-
Displacement of barium from barium hydroxide and iron from iron chloride –
$\mathrm{FeCl}_3+\mathrm{Ba}(\mathrm{OH})_2 \rightarrow \mathrm{Fe}(\mathrm{OH})_2+\mathrm{BaCl}_2$
-
Displacement of sodium from sodium sulphate and lead from lead nitrate –
$\mathrm{Pb}\left(\mathrm{NO}_3\right)_2+\mathrm{Na}_2 \mathrm{SO}_4 \rightarrow \mathrm{PbSO}_4+2 \mathrm{NaNO}_3$
- Displacement of barium from barium chloride and copper from copper sulphate –
$\mathrm{BaCl}_2+\mathrm{CuSO}_4 \rightarrow \mathrm{BaSO}_4+\mathrm{CuCl}_2$
Also read -
Some Solved Examples
Question 1: Match the following reactions to their types:
1. $2 \mathrm{AgBr} \xrightarrow{\text { sunlight }} 2 \mathrm{Ag}+\mathrm{Br}_2$ p. Combination reaction
2.$3 \mathrm{MnO}_4^{2-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2$ q. Disproportionation reaction
3.$2 \mathrm{Na}+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{Na}_2 \mathrm{O}+2 \mathrm{H}_2 \mathrm{O}$ r. Decomposition reaction
4.$\mathrm{CuSO}_4+\mathrm{Zn} \rightarrow \mathrm{ZnSO}_4+\mathrm{CuS}$ s. Non-metal displacement reaction
1) (correct) $1-r, 2-q, 3-p, 4-s$
2) 1-q,2-s,3-p,4-r
3) 1-q,2-p,3-s,4-r
4) 1-s,2-q,3-p,4-r
Solution:
As we learnt
Decomposition Reaction -
This is opposite of combination reaction precisely, a decomposition reaction lead to the breakdown of a compound into two or more components at least one of which must be in an elemental state.
- wherein
$2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \stackrel{\Delta}{\rightarrow} 2 \mathrm{H}_2(g)+\mathrm{O}_2(g)$
+1 -2 0 0
$2 \mathrm{AgBr} \xrightarrow{\text { sunlight }} 2 \mathrm{Ag}+\mathrm{Br}_2$ is a decomposition reaction.
Hence, the answer is the option (1).
Question 2: Which of the following is non metal displacement Redox Reaction.
1) $\mathrm{Mg}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_2+\mathrm{H}_2$
2) $2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{~F}_2 \rightarrow 4 \mathrm{HF}+\mathrm{O}_2$
3) $\mathrm{Cl}_2+2 \mathrm{KI} \rightarrow 2 \mathrm{KI}+\mathrm{I}_2$
4) (correct) All of these
Solution:
As we learned
Non-metal Displacement -
The non-metal displacement redox reaction includes hydrogen displacement and a rarely occurring reaction involving oxygen displacement.
- wherein
$2 \mathrm{Na}(\mathrm{s})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow 2 \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})$
0 +1 -2 1 -2 +1 0
Given all non-metals are displacement Redox reaction.
Hence, the answer is the option (4).
Question 3: Which of the following is Metal displacement Redox Reaction.
1) $2 \mathrm{Na}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_2$
2) (correct) $\mathrm{CuSO}_4+\mathrm{Zn} \rightarrow \mathrm{ZnSO}_4+\mathrm{Cu}$
3) $\mathrm{Cl}_2+2 \mathrm{OH}^{-} \rightarrow \mathrm{ClO}^{-}+\mathrm{Cl}^{-}+\mathrm{H}_2 \mathrm{O}$
4) $3 M g+N_2 \rightarrow M g_3 N_2$
Solution:
As we learned
Metal Displacement Reaction -
A metal in a compound can be displaced by another metal in the uncombined. It has much application in metallurgical processes.
- wherein
$V_2 \mathrm{O}_5(\mathrm{~s})+5 \mathrm{Ca}(\mathrm{s}) \xrightarrow{\Delta} 2 \mathrm{~V}(\mathrm{~s})+5 \mathrm{Ca} \mathrm{O}(\mathrm{s})$
+5 -2 0 0 +2 -2
Metal displacement Redox Reaction is
$\mathrm{CuSO}_4+\mathrm{Zn} \rightarrow \mathrm{ZnSO}_4+\mathrm{Cu}$
Hence, the answer is the option (2).
Question 4: In which of the following cases a chemical reaction is possible?
1) (correct) $\mathrm{AgNO}_3$ solution is stirred with a copper spoon
2) Conc. $\mathrm{HNO}_3$ is stored in a platinum vessel
3) gold ornaments are washed with dil HCl
4) $\mathrm{ZnSO4}(\mathrm{aq})$ is placed in a copper vessel
Solution:
When AgNO3 solution is stirred with a copper spoon, displacement reaction will take place which will form a new compound called as copper nitrate.
In all other cases no chemical reaction takes place between the components taken.
Hence only option in which chemical reaction is possible is when AgNO3 solution is stirred with a copper spoon
Hence, the answer is the option (1).
Practice more question with the link given below
Frequently Asked Questions (FAQs)
A replacement reaction or displacement reaction also known as exchange reaction is a reaction in which a higher reactive element displaces the lower reactive element from the compound. Single Replacement reactions are of three types- metal replacement, halogen replacement and hydrogen replacement. Examples:
2Ag+H2S→Ag2S+H2(Hydrogen replacement)
When one element in a compound is replaced by another, it is called a single displacement reaction. A metal can only replace another metal, and a nonmetal can only replace another nonmetal.
In double substitution reactions, two ionic compounds exchange anions or cations. When the reactants are acid and base, neutralisation reactions occur, and neutralisation reactions are generally favourable as long as the reaction demands a solid acid and/or base.
Metal replacement reaction is a reaction in which a metal is displaced from another metal, which is more reactive than it. Example: Mg+Cu(NO3)2 →Mg(NO3)2+Cu
Magnesium is a metal with a higher reactivity than copper. When a strip of magnesium metal is immersed in aqueous copper (II) nitrate solution, it replaces the copper. The process produces aqueous magnesium nitrate and solid copper metal as byproducts.
Because the halogens gain electrons and the halide ions lose electrons, halogen displacement reactions are redox reactions.
We can see which element is being oxidised and which is being reduced when we study one of the displacement reactions. Example: Br2+2KI→I2+2KBr
Bromine has gained electrons, indicating that it has been reduced. The iodide ions have been oxidised because they have lost electrons.