Electronegativity - Overview, Factors, Elements, Applications, FAQs
Electronegativity is another important concept within chemistry that provides insight into how the atomic structures of the different elements manage to attract some electrons when both of them are involved in a chemical bond. This idea was earlier formulated by Linus Pauling; electronegativity reflects how many electrons an element attracts toward its nucleus during the performance of a chemical bond. This property determines the characteristics of the bonding— it can be ionic, covalent, or polar covalent, and describes the distribution of charge within the atoms of the molecule. Since fluorine or oxygen has a higher electronegativity than the other elements, it attracts the electrons more sharply creating a strong polar covalent bond in which the electron is found more in the vicinity of fluorine or oxygen atom.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Unveiling the Power of Electronegativity
- Some Solved Examples
- Conclusion
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
In this article, we will be focussing on the in-depth knowledge of the important topic of Electronegativity, which is the sub-topic of the chapter Classification of Elements and Periodic Table from class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more. Over the last ten years of the JEE exam (from 2013 to 2023), three questions have been asked on this concept, a total of twelve questions have been asked from this concept, and eight questions in JEE from 2013 to 2023.
Related Topics Link
- Electronic Configuration of First 30 Elements
- Electron Gain Enthalpy
- Homologous Series
- Atomic radius in periodic table in basic chemistry
- Classification of Elements in Modern Periodic Table
- 118 elements, their symbols and their atomic number
Unveiling the Power of Electronegativity
Electronegativity
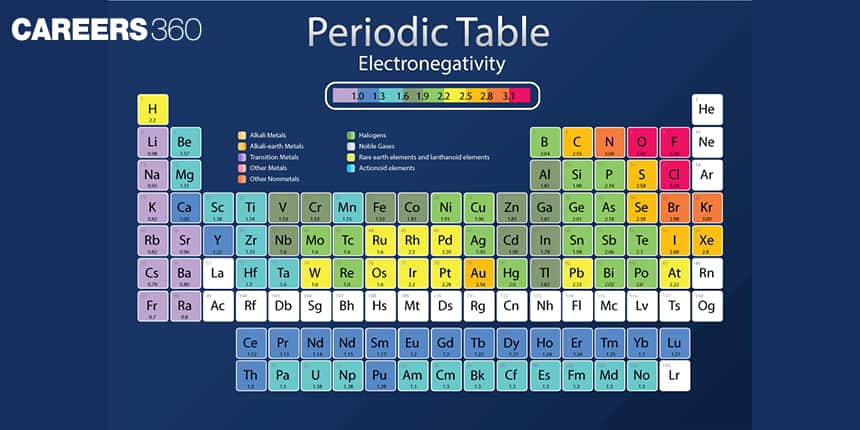
The tendency of an atom to attract the shared pair of electrons towards itself is called electronegativity. It is a relative quantity. This concept was introduced in 1932 by Pauling. It has no units. Fluorine is the most electronegative element known so far and its value is arbitrarily assigned as 4.0. In moving from left to right in a period, the electronegativity increases while in moving from the top to bottom in a group, the electronegativity decreases.
Factors affecting Electronegativity
There are various factors which affect the electronegativity.
Atomic Size: As the atomic size increases, the electronegativity decreases.
Effective Nuclear Charge: With the increase of effective nuclear charge, the electronegativity of the atom increases.
Oxidation State: As the oxidation state of an atom increases, the electronegativity also increases. For example, Fe3+ is more electronegative than Fe2+.
Read more :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Recommended topic video on (Electronegativity)
Some Solved Examples
Example 1: The correct option concerning the Pauling electronegativity values of the elements is :
1) Ga < Ge
2) P > S
3) Si < Al
4) Te > Se
Solution: Electronegativity -A qualitative measure of the ability of an atom in a chemical compound to attract shared electrons is electronegativity.- wherein It is not a measurable quantity.
Electronegativity and non-metallic character -
Non-metallic elements have a strong tendency to gain electrons. Therefore electronegativity is directly related to non-metallic properties of elements.
- wherein
Electronegativity ∝ non-metallic property
Correct order
(1) Ga<Ge
(2) Si<Al
(3) P<S
(4) Te<Se
Hence, the answer is the option (1).
Example 2: The electronegativity of an element is related to ionization energy and
1) Atomic radii
2) Electron affinity
3) Ionic radii
4) Nucleus
Solution: The electronegativity depends upon the sum of Ionisation and Electron Affinity.
The electronegativity of any given element is not constant. It varies depending on the elements to which it is bound.
Though it is not measurable, it does provide a means to predict the nature of the force that holds atoms.
More electronegative elements will have positive Electron affinity due to electron attraction as well as more Ionisation Energy.
Hence, the answer is the option (2).
Example 3: Which of the following is the most electronegative?
1) Be
2) B
3) C
4) (correct) N
Solution: Electronegativity generally increases across a period from left to right.
e.g. from lithium to fluorine.
N is the most electronegative element among the given elements as we move from left to right in a period, the electronegativity increases.
Hence, the answer is the option (4).
Example 4: Which one of the following elements is most electronegative?
1) Fluorine
2) Sulphur
3) Oxygen
4) Bromine
Solution: As we learned, Variation of electronegativity along group
Electronegativity generally decreases down a group in the periodic table.
- wherein
e.g. from fluorine to astatine.
Electronegativity decreases as we move down the group and increases as we move from left to right in a period.
Hence, the answer is the option (1).
Example 5: Which of the following is most electronegative?
1) (correct) Carbon
2) Silicon
3) Lead
4) Tin
Solution: Electronegativity - The electronegativity of any element decreases down the group. Thus carbon is the most electronegative element.
Hence, the answer is the option (1).
Variation of Electronegativity
In moving from top to bottom in a group the atomic size increases thus the force of attraction decreases and hence the electronegativity decreases.
In moving from left to right in a period, the atomic size decreases and effective nuclear charge increases, thus the electronegativity increases.
Halogens are the most electronegative elements and fluorine has the highest electronegativity.
For transition elements, the electronegativity values vary between 1.1 to 1.3.
Metals have lower electronegativity values while non-metals have higher electronegativity values.
Example 6: Two elements with electronegativities are 1.2 and 3.2 respectively, the bond formed between them will be:
1) Covalent
2) Metallic
3) Ionic
4) None
Solution: Electronegativity - Nature of Bond: The nature of the bond can be estimated from the electronegativity values of respective atoms.
(i) When the electronegativity difference between two atoms, i.e., MA—MB = 0, then the bond is purely covalent.
(ii) When MA - MB is small, the bond is polar but covalent.
(iii) When MA - MB is 1.9, the bond is 50% ionic and 50% covalent.
(iv) When MA—MB is greater than 1.9, the bond is more ionic and less covalent.
The percentage of ionic character is given by the following formula:
Percentage of ionic character = 16(MA - MB) + 3.5(MA - MB)2
MA and MB are the electronegativities of two bonded atoms, i.e., A and B.
The electronegativity difference between the constituent atoms must be greater than 1.9 to form the ionic bond.
Hence, the answer is the option (3).
Example 7: On going from right to left in a period in the periodic table the electronegativity of the elements
1) Increases
2) Decreases
3) Remain unchanged
4)Decreases first then increases
Solution: As we learned, Electronegativity is the ability of any atom to attract a bonded pair of electrons towards itself. It increases on moving from left to right along a period as the size of the atom decreases.
Hence, the answer is the option (1).
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Importance of Electronegativity
The following predictions can be made out of the information on the electronegativities of atoms.
Nature of Element: The elements with lower electronegativity values are metals while the elements with higher electronegativity values are non-metals. The elements with intermediate electronegativity values are metalloids. Fluorine has the highest electronegativity value, thus it is the most non-metallic element. Similarly, cesium has the lowest electronegativity value, thus it is the most metallic element.
- Nature of Oxides: The nature of the oxides formed by the elements can also be predicted by electronegativity. When the MO - MA difference is lower, then the oxide is acidic but when this difference MO - MA is large, then the oxide is basic. MO here is the electronegativity of oxygen.
Conclusion
Therefore, electronegativity is a versatile concept that defines the formation of chemistry as well as its interaction with the world. It gives a chemical method of determining an element’s electron-capturing capacity within a given chemical bond hence affecting the nature of bonding and the nature of the resultant matter formation. Those with higher electronegativity values attract more electrons to their side than the other element and thereby result in the formation of polar bonds and the extent of polarity of a molecule. This property is of paramount importance in so many processes in chemistry among them including the behaviour of acids and bases as well as solubility of substances in different solvents.
Frequently Asked Questions (FAQs)
Electronegativity increases across a period because number of charges on nucleus increases. As a result, the bonding pair of electrons is attracted more strongly.
Electronegativity of fluorine has the highest electronegativity.
Electronegativity of H is 2.20.
Electronegativity of C is 2.55.
Electronegativity of N is 3.04.
Electronegativity of O is 3.44.
The atomic number increases as we proceed down the group. The nuclear charge increases as well, but the effect of the increase is mitigated by the addition of one shell. As a result, as we travel down the group, the value of electronegativity decreases.
Here we must find which element has the highest electronegativity for that in a group, electronegativity decreases as the size increases, leading to its ability to attract electrons decrease. Thus, P<N and Si<C. In a period, as the size decreases, electronegativity increases due to the increase in effective nuclear charge. Thus, C<N and P>Si.
Hence, the overall order is Si<P<C<N Therefore, N has highest electronegative element.
Also Read
12 Mar'25 09:34 AM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
09 Jan'25 03:51 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM