Hello,
The formula of equilibrium constant depends on the reaction.
For a general reaction:
aA + bB = cC + dD
The equilibrium constant (Kc) is:
Kc = (C power c × D power d) / (A power a × B power b)
Here, the concentrations of products are in
Equilibrium - Notes, Topics, Formula, Books, FAQs
Equilibrium is reached when the forward and reverse reactions happen at the same rate, so the amounts of reactants and products stay constant. It’s a dynamic state reactions continue, but there's no overall change. Think of it like pouring from one bucket to another at the same rate in both directions you are pouring, but the water levels don’t change. Equilibrium comes in two forms: chemical equilibrium, which involves substances transforming in chemical reactions, and ionic equilibrium, where dissolved molecules and ions in a solution balance each other. Though it appears stable, underlying activity continues on both sides.
This Story also Contains
- Important Topics Of Chemical Equilibrium
- Overview Of Chemical Equilibrium
- Equilibrium In Physical Process
- Applications of Equilibrium Constant
- Reaction Quotient (Q):
- Factors Affecting Chemical Equilibrium
- Ionic Equilibrium in Solution
- Important Topics Of Ionic Equilibrium
- Overview Of Ionic Equilibrium
- How to prepare for Equilibrium?
- Equilibrium in Different Exams:
- Study Links for Equilibrium
- Subject Wise Resources of NCERT
- Prescribed Books for Equilibrium
- Previous Year Question Of Equilibrium
- Conclusion
.jpg)
Important Topics Of Chemical Equilibrium
This chapter deals with the concept of reversible reactions and the conditions under which equilibrium is established. It focuses on equilibrium constants, Le Chatelier’s principle, and factors affecting equilibrium position. These concepts are essential for understanding reaction behaviour in Chemistry.
Law Of Mass Action
For a reaction like $\mathrm{aA}+\mathrm{bB} \rightarrow$ products, the rate depends on $[A]^a \times[B]^b$, where “a” and “b” are the amounts shown in the reaction’s equation and [A], [B] are their concentrations.The Law Of Mass Action states that the speed of a reaction is proportional to how much of each reactant is present.
In equilibrium, the forward and reverse reactions balance out, and the ratio of product and reactant activities stays constant. This principle, formulated by Guldberg and Waage in the 1860s, applies best to simple (elementary) reactions and helps define the familiar equilibrium constant expression.
Equilibrium Constant:
Equilibrium Constant is the ratio of the rate of forward and backward reaction at a particular temperature or it is the ratio of active masses of the reactants to that of active masses of products at a particular temperature raised to their stoichiometric coefficients.
Relation Between $\mathrm{K}_{\mathrm{p}}$ And $\mathrm{K}_{\mathrm{c}}$:
The relationship between the concentrations or partial pressures of reactants and products in a chemical reaction at equilibrium is called Relation Between Kp And Kc.
$K_c-$ Equilibrium constant in terms of concentration $\left(\mathrm{mol} \mathrm{L}^{-1}\right)$
For a general gaseous reaction
$$\begin{gathered}
a A+b B \rightleftharpoons c C+d D \\
K_c=\frac{[C]^c[D]^d}{[A]^a[B]^b}
\end{gathered}$$
$K_p$ - Equilibrium constant in terms of partial pressure (atm or bar)
$$K_p=\frac{\left(P_C\right)^c\left(P_D\right)^d}{\left(P_A\right)^a\left(P_B\right)^b}$$
So,
$$K_p=K_c(R T)^{\Delta n}$$
Where
$$\Delta n=(c+d)-(a+b)$$
Degree Of Dissociation:
Degree Of Dissociation is the extent to which any reactant gets dissociated. Due to dissociation, the total number of moles at equilibrium can be determined. Knowing the number of moles at equilibrium, the observed molar mass can be calculated.
Le Chatelier's Principles On Equilibrium:
It is defined as the ratio of the concentration of products to the concentration of the reacting species raised to their stoichiometric coefficient at any point of time other than the equilibrium stage is called Le Chatelier's Principles On Equilibrium.
Overview Of Chemical Equilibrium
This section gives a brief introduction to the concept of equilibrium in chemical reactions, highlighting how opposing reactions occur simultaneously and reach a state of balance.
Take a reaction as follows:
$p P+q Q \rightleftharpoons r R+s S$
Equilibrium Constant is given by the following equation:
$\mathrm{Kc}=[\mathrm{P}]^{\mathrm{p}}[\mathrm{Q}]^{\mathrm{q}}[\mathrm{R}]^{\mathrm{r}}[\mathrm{S}]^{\mathrm{S}}$
Chemical equilibrium occurs when both the reactions(forward and backward) are occurring at equal rates.
The chemical equilibrium and its laws have various real-life applications as mentioned below:
-
Haemoglobin and oxygen exist in equilibrium in the blood by the reaction:
$$\mathrm{Hb}(\mathrm{aq})+4 \mathrm{O}_2 \rightleftharpoons \mathrm{Hb}\left(\mathrm{O}_2\right)_4(\mathrm{aq}) .$$
As a result, a person tends to feel light-headed at higher altitudes.
-
Nitrogen and other gases are dissolved in our blood, now if the diver comes up too fast from the deep ocean, suddenly there is an equilibrium shift takes place thus develops nitrogen bubbles in the blood which causes severe pain and death.

Equilibrium In Physical Process
Some of the phase processes of equilibrium are as follows:
-
Solid-liquid equilibrium:
For example, ice and water are at equilibrium at only one temperature is called a normal melting point. -
Liquid-gas equilibrium:
For example, rate of evaporation = rate of condensation$\mathrm{H}_2 \mathrm{O}(\mathrm{I}) \rightleftharpoons \mathrm{H}_2 \mathrm{O}($ vap $)$ -
Solid-gas equilibrium:
This process is also called sublimation
e.g. sublimation of camphor
Solid-liquid equilibrium:
Law of mass action: The equilibrium concentrations of reactants and products may vary, but the value $\mathrm{K}_{\mathrm{C}}$ remains constant.
Characteristics of $\mathrm{K}_{\mathrm{C}}$
-
The reaction equilibrium can be obtained from both directions.
-
$\mathrm{K}_{\mathrm{C}}$ is a function of temperature.
-
$\mathrm{K}_{\mathrm{C}}$ is independent of the initial concentration of reactants and products.
-
If the $\mathrm{K}_{\mathrm{C}}$ value is
-
<< 1, the equilibrium lies to the right and the reaction mixture contains mostly products.
-
0.10<$\mathrm{K}_{\mathrm{C}}$<10, the mixture contains a proportionate amount of reactants and products.
-
>> 1, the equilibrium lies to the left and the reaction mixture contains mostly reactants.
Homogeneous Equilibria
When all the reactants and the products in the equilibrium are in the same phase, then this equilibrium is known as homogeneous equilibrium. For example:
$\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_3(\mathrm{~g})$
In this reaction, all these reactants are in the gaseous phase, thus it is a homogeneous equilibrium
Heterogeneous Equilibria
When the reactants are in different phases, then this system of equilibrium is known as the heterogeneous equilibrium. For example,
$\mathrm{Ca}(\mathrm{OH})_2(\mathrm{~s}) \rightleftharpoons \mathrm{Ca}^{2+}(\mathrm{aq})+2 \mathrm{OH}^{-}(\mathrm{aq})$
In this reaction, all these reagents are in different phases, thus it is a heterogeneous equilibrium.
Applications of Equilibrium Constant
- Predicts the extent of reaction, which gives the degree of the disappearance of reactants.
- Predicts the direction of the reaction.
- Calculating the equilibrium constant, which gives the relative amount of reactants and products.
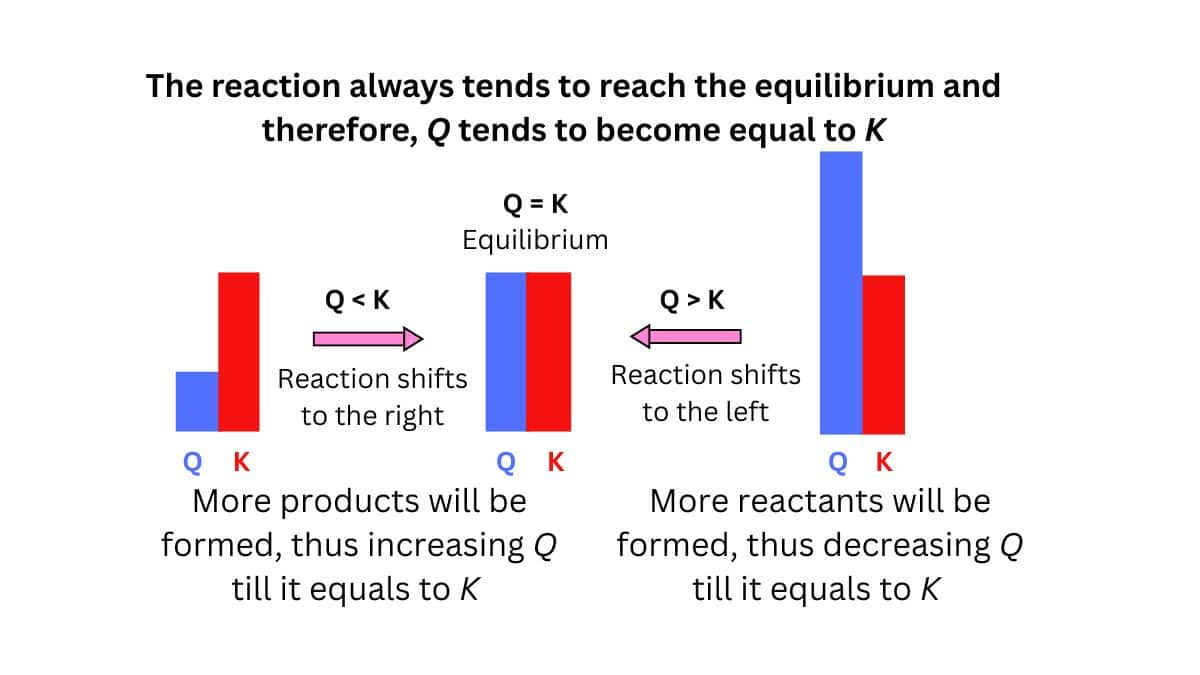
Reaction Quotient (Q):
The reaction value obtained when we substitute reactant and product concentrations into the equilibrium expression.
-
If Q>K the reaction shifts towards the reactants side.
-
If Q<K the reaction shifts towards the products side.
-
If Q+K no shift occurs (equilibrium achieved).
Factors Affecting Chemical Equilibrium
- Lechatlier’s Principle: Le Chatelier’s Principle explains how a system at equilibrium responds to changes. If temperature, pressure, volume, or concentration is changed, the system adjusts itself to reduce the effect of that change and reach a new balance. For example, if more of a reactant is added, the reaction will shift to make more product. This principle helps us understand and control reactions, and is useful in choosing the best conditions to produce a desired substance efficiently.
- Changes in temperature:
Endothermic(H>0): R+ Heat → Products.
Exothermic(H<0): R→ Products+ Heat - Effect of catalyst:
The Catalyst increases the rate of reaction for both forward and reverse reactions. The catalyst does not affect the equilibrium concentration of both reactants and products. So the value $\mathrm{K}_{\mathrm{C}}$ doesn’t change.
Ionic Equilibrium in Solution
Ionic equilibrium refers to a balance in solutions where ions and their undissociated molecules coexist. Substances that dissolve into ions are called electrolytes. Strong electrolytes like soluble salts, strong acids, and bases dissociate completely and conduct electricity well. Weak electrolytes such as acetic acid or ammonia only partially ionize, so they conduct weakly and show a reversible equilibrium between ions and molecules.This concept explains how acids, bases, and salts behave in water and is key to understanding solution chemistry.
Important Topics Of Ionic Equilibrium
Bronsted Lowry And Lewis Acid-base Theory:
According to Bronsted Lowry And Lewis Acid-base Theory, an acid is a substance that can donate a proton and a base is a substance that can accept a proton
Ionization Of Acids And Bases:
The process by which a neutral molecule breaks down into charged ions when exposed to a solution is known as compound ionization. When an acid dissociates into an aqueous medium to produce the hydrogen ion $\mathrm{H}^{+}$ and bases are the hydroxide compounds that give $\mathrm{OH}^{-}$ ions on dissociation in water called Ionization Of Acids And Bases
$\mathrm{K}_{\mathrm{a}}$ And $\mathrm{K}_{\mathrm{b}}$ Relationship:
Ka and Kb are equilibrium constants that indicate the relative amounts of products and reactants at equilibrium.
Consider a weak acid HA:
$\begin{gathered}
H A+H_2 O \rightleftharpoons H_3 O^{+}+A^{-} \\
K_a=\frac{\left[H_3 O^{+}\right]\left[A^{-}\right]}{[H A]}
\end{gathered}$
Its conjugate base $\mathrm{A}^{-}$reacts as a weak base:
$\begin{gathered}
A^{-}+H_2 O \rightleftharpoons O H^{-}+H A \\
K_b=\frac{\left[O H^{-}\right][H A]}{\left[A^{-}\right]}
\end{gathered}$
Multiply $K_a$ and $K_b$ :
$K_a \times K_b=\frac{\left[H_3 O^{+}\right]\left[A^{-}\right]}{[H A]} \times \frac{\left[O H^{-}\right][H A]}{\left[A^{-}\right]}$
Hence,
$K_a \times K_b=K_w$
Buffer Solution
A solution whose pH does not change very much when $\mathrm{H}^{+}\left(\mathrm{H}_3 \mathrm{O}^{+}\right)$or $\mathrm{OH}^{-}$ are added to it is referred to as a Buffer Solution.
Common Ion Effect:
The Common Ion Effect is defined as the phenomenon where the solubility of an ionic compound decreases in a solution that already contains one of the ions present in the compound.
PH Of Acids And Bases:
A solution having a PH between 0 to 7 is called an acid and a solution whose PH ranges in between 7 and above up to 14 is called a base and the pH in between 0 to 14 is called Ph Of Acids And Bases.
Solubility And Solubility Product:
The maximum amount of a particular solute in grams, which can dissolve in 100 grams of solvent at a given temperature is called Solubility And Solubility Product is the product of the molar concentrations of ions of an electrolyte in a saturated solution at a particular temperature.
Salt Hydrolysis:
When a salt is added to water ions of the salt interact with water to cause acidity or basicity in an aqueous solution. This ionic interaction is called Salt Hydrolysis.
Overview Of Ionic Equilibrium
This section introduces the equilibrium established between ions in solution, explaining concepts such as ionisation, pH, pOH, and the behaviour of acids, bases, and salts in aqueous chemistry.
Ionization of Acids and Bases
As already mentioned, some acids and bases are strong in nature and some are weak in nature. When a strong acid dissociates in the aqueous medium then it forms its conjugate base, these conjugate bases are always weak in nature. Similarly, with strong bases, their conjugate acids in solutions are always weak. For weak acids and bases, their conjugates bases and acids, respectively are always strong in nature.
The acid-base dissociation equilibrium of an acid HA is given as below:
$\mathrm{HA}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}+\mathrm{A}^{-}(\mathrm{aq})$
an acid-base conjugate acid conjugate base
Ionic Product of Water
In pure water, one water molecule donates a proton and acts as an acid and another water molecule accepts a proton and acts as a base. The equilibrium reaction goes as follows:
$\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightleftharpoons \mathrm{H}_3 \mathrm{O}^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})$
The dissociation constant for this equilibrium reaction is given as follows:
$\mathrm{K}=\left[\mathrm{H}_3 \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right] /\left[\mathrm{H}_2 \mathrm{O}\right]$
[H2O] can be eliminated since it is liquid, thus the ionic product of water or Kw is given as:$\mathrm{K}=\left[\mathrm{H}_3 \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]$
Thus any aqueous solution is acidic, basic, or neutral is determined by the conditions below:
- For acidic: $\left[\mathrm{H}_3 \mathrm{O}^{+}\right]>\left[\mathrm{OH}^{-}\right]$
- For neutral: $\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\left[\mathrm{OH}^{-}\right]$
- For basic: $\left[\mathrm{H}_3 \mathrm{O}^{+}\right]<\left[\mathrm{OH}^{-}\right]$
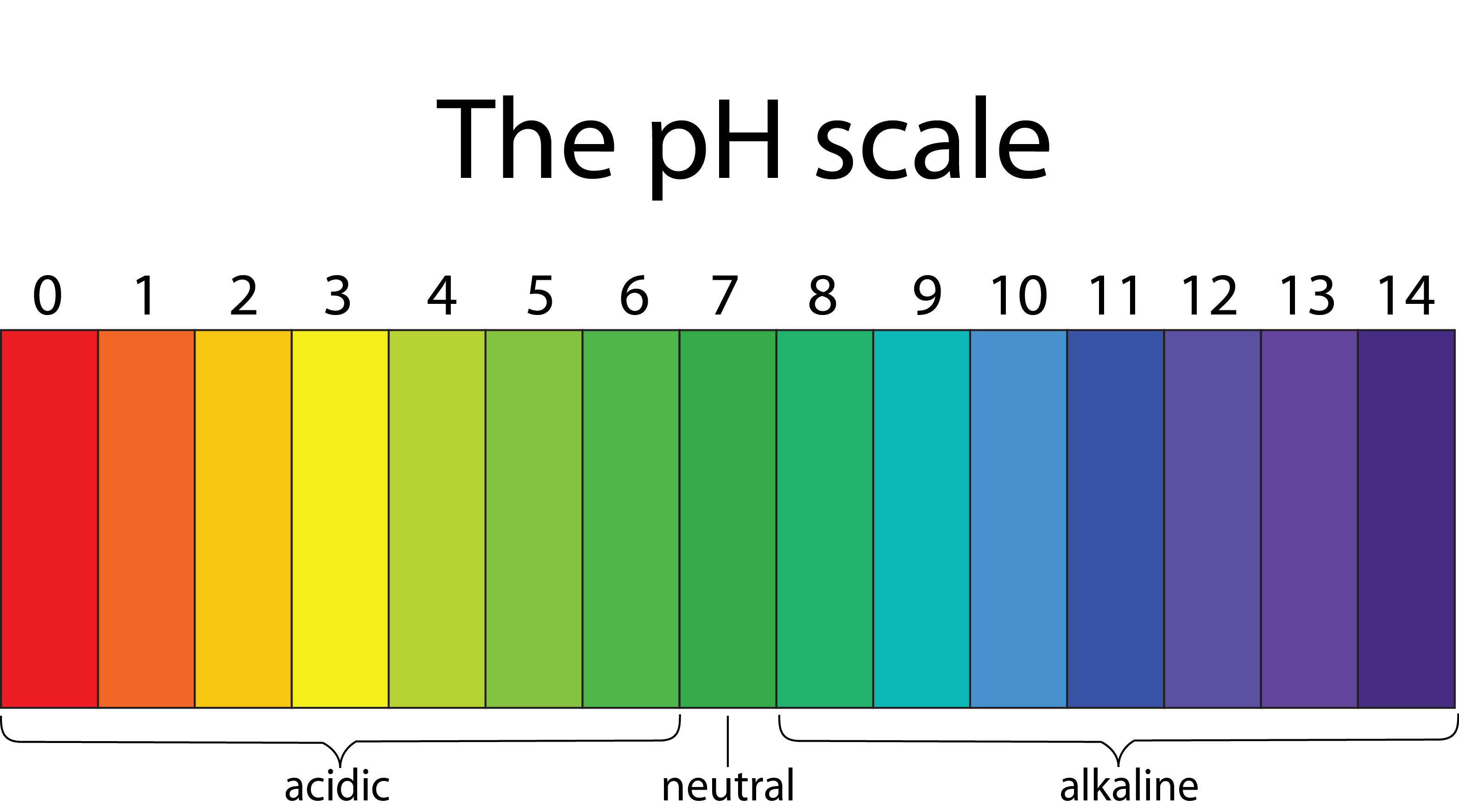
The pH Scale
The hydronium ion concentration of any solution is expressed in a logarithmic scale, then it is known as pH scale. Thus, the pH of any solution is defined as the negative logarithm value of hydrogen ion concentration. Mathematically, it can be expressed as follows:
$\mathrm{pH}=-\log _{\mathrm{a}}\left(\mathrm{H}^{+}\right)$
Now as we know, the pure water has hydrogen ion concentration = $10^{-7}$, therefore, pH of water = 7
Thus we can conclude as follows:
- Acidic solution has pH < 7
- Basic solution has pH > 7
- Neutral solution has pH = 7
From the above calculations, it has been found that:
pKw=pH+pOH=14
The relation between $\mathrm{K}_{\mathrm{a}}$ and $\mathrm{K}_{\mathrm{b}}$
$\mathrm{K}_{\mathrm{a}}$ and $\mathrm{K}_{\mathrm{b}}$ are the strengths of acidic and basic solutions respectively. Now, for every net reaction, the equilibrium constant is equal to the product of the added reactions.
Thus, $\mathrm{K}_{\mathrm{a}} \times \mathrm{K}_{\mathrm{b}}=\mathrm{K}_{\mathrm{w}}$
Therefore, $\mathrm{pK}_{\mathrm{a}}+\mathrm{pK}_{\mathrm{b}}=\mathrm{pK}_{\mathrm{w}}=14$
Buffer Solutions
These are the solutions that resist the change in pH if the solution is diluted or some small amounts of alkali or acid are added. For example, a mixture of acetic acid and sodium acetate is a buffer solution with pH equal to 4.75.
How to prepare for Equilibrium?
This chapter of Physical Chemistry is one of the most essential in the entire chemistry syllabus. Understanding its theories, principles, formulas, and graph interpretations is key to building a strong foundation in chemistry.
- Mastery of these topics not only enhances conceptual clarity but also boosts your exam scores. Whether you're solving equilibrium problems, applying laws like Le Chatelier’s, or interpreting graphs, each concept plays a vital role.
- Studying this chapter thoroughly equips you with analytical tools that are tested across multiple areas of chemistry and are critical for academic success.
-
Before reading this chapter, first, you must have a basic knowledge of the mole concept.
-
In this chapter, all the equations and formulae are very simple and easy to remember.
-
Rest this chapter is very simple, just be regular and be consistent in your numerical practice.
Equilibrium in Different Exams:
This chapter is frequently asked in board and competitive examinations with emphasis on chemical and ionic equilibrium, equilibrium constants, and problem-solving involving acids, bases, and salts.
| Exam Name | Focus Area | Common Topics Asked | Preperation Tips |
| CBSE Board | Conceptual understanding | Chemical & ionic equilibrium, equilibrium constants | Practise NCERT examples and numericals |
| JEE Main | Numerical problem-solving | Kc, Kp, pH, buffer solutions | Focus on formula application |
| JEE Advanced | Analytical understanding | Complex equilibrium problems | Strengthen conceptual clarity |
| NEET | NCERT-based theory | pH calculations, acids and bases | Revise NCERT thoroughly |
| State Board Exams | Theory-oriented | Definitions, equilibrium laws | Learn key concepts and terms |
| Chemistry Olympiads | Advanced application | Buffer solutions, solubility product | Practise high-level numerical problems |
Study Links for Equilibrium
This section provides useful reference links and study materials to help understand chemical and ionic equilibrium concepts, numerical problems, and their applications in chemistry.
Subject Wise Resources of NCERT
This section provides organised study materials and useful links for each subject to support effective learning and exam preparation.
Prescribed Books for Equilibrium
First, you must finish the class XI NCERT book and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and O.P. Tandon. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously write the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Previous Year Question Of Equilibrium
This section includes important questions asked in previous examinations that focus on both chemical and ionic equilibrium concepts, helping students understand exam patterns and frequently tested topics.
Question 1: With increase in temperature $\mathrm{K}_{\mathrm{w}}$ increases . If at a certain temperature $\mathrm{K}_{\mathrm{w}}$ is $10^{-12}$ then a solution of pH = 6.5 will be:
1) Acidic
2) Basic
3) Neutral
4) Insufficient information
Answer:
As we learned
If $K_W=10^{-12}$ then pH = 6 or pOH = 6 solution will be neutral and at pH = 6.5 solution will be basic.
Hence, the answer is the option (2).
Question 2: Consider the equilibria (1) and (2) with equilibrium constants $\mathrm{K}_1$ and $\mathrm{K}_2$ respectively:
& \mathrm{SO}_2(g)+1 / 2 \mathrm{O}_2(g) \rightleftharpoons \mathrm{SO}_3(\mathrm{~g}) \\
& 2 \mathrm{SO}_3(\mathrm{~g}) \rightleftharpoons 2 \mathrm{SO}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \ldots
\end{aligned}$$
$K_1$ and $K_2$, are related as
1) $2 K_1=K_2^2$
2) $K_1^2=\frac{1}{K_2}$
3)$K_2^2=\frac{1}{K_1}$
4) $K_2=\frac{2}{K_1^2}$
Answer:
$\left.\mathrm{SO}_2(g)+\frac{1}{2} \mathrm{O}_{[2} 2(g) \rightleftharpoons \mathrm{SO}_3(g)-K_{} 1\right]$
$2 S_3(g) \rightleftharpoons 2 \mathrm{SO}_2(g)+\mathrm{O}_2(g) \mathrm{K}_2$
$\begin{aligned}
& K_1=\frac{\left[\mathrm{SO}_3\right]}{\left[\mathrm{SO}_2\right]^2\left[\mathrm{O}_2\right]^{\frac{1}{2}}} \\
& K_2=\frac{\left[\mathrm{SO}_2\right]^2\left[\mathrm{O}_2\right]}{\left[\mathrm{SO}_3\right]^2}=\left(\frac{1}{K_1}\right)^2
\end{aligned}$
$K_1^2=\frac{1}{K_2}$
Hence, the answer is the option (2).
Question 3: For the reaction $N_2 O_4(g) \leftrightharpoons 2 \mathrm{NO}_2(g)$, , the value of K is 50 at 400 K and 1700 at 500 K. Which of the following options is correct?
(i) The reaction is endothermic.
(ii) The reaction is exothermic.
(iii) If $\mathrm{NO}_2(\mathrm{~g})$ and $\mathrm{N}_2 \mathrm{O}_4(\mathrm{~g})$ are mixed at 400 K at partial pressures, 20 bar and 2 bar respectively, more $N_2 O_4(g)$ will be formed.
(iv) The entropy of the system increases.
1) (i), (ii) and (iv)
2) (ii) and (iv)
3) (ii) and (iii)
4) (i), (iii) and (iv)
Answer:
The answer is the option (i), (iii) and (iv)
Explanation: K increases with the increase in temperature, and when K increases, the reaction must be endothermic. Thus, the number of molecules increases, and there is an increase in entropy.
Hence, the answer is the option (4).
Practice more questions from the link given below:
Conclusion
Equilibrium describes a dynamic balance where forward and reverse reactions proceed at equal rates, keeping concentrations steady. Understanding the equilibrium constant (K), reaction quotient (Q), and Le Chatelier’s principle helps predict and influence reaction behavior. These concepts are essential in industries (e.g., Haber and Contact processes), biology (like buffer systems), and environmental chemistry. In exams like JEE Main, NEET, and BITSAT, equilibrium typically features 1–2 high-scoring questions (about 6-8% weight in JEE Main) making strong grasp vital for competitive success.
Frequently Asked Questions (FAQs)
A reversible reaction is a chemical reaction where the products can react to re-form the original reactants. These reactions are typically represented by a double arrow ($\rightleftharpoons$) between reactants and products.
- Dynamic Nature: Both forward and reverse reactions occur at equal rates.
- Constant Macroscopic Properties: Observable properties like temperature, pressure, concentration, and color remain constant.
- Achievable from Either Direction: Equilibrium can be reached whether starting with pure reactants or pure products.
- Closed System: Equilibrium can only be established in a closed system where no matter can enter or leave.
- Effect of Catalyst: A catalyst speeds up both forward and reverse reactions equally, helping to reach equilibrium faster but not changing the equilibrium position.
Inert salts (e.g., NaClO₄, KNO₃) help maintain ionic strength without influencing specific equilibrium reactions.
It helps determine how the equilibrium constant varies with temperature. The slope of a Van ’t Hoff plot reveals enthalpy and entropy changes.
Le Chatelier’s Principle states that if a dynamic equilibrium is disturbed by changing the conditions (such as concentration, temperature, or pressure), the system will respond by shifting the equilibrium position to counteract the change and restore a new equilibrium. For example, if the concentration of a reactant is increased, the system will shift to favor the formation of products.
Several factors can affect chemical equilibrium, including:
- Concentration: Changing the concentration of either reactants or products can shift the equilibrium position.
- Temperature: In endothermic reactions, increasing temperature shifts equilibrium to favor products, while in exothermic reactions, it favors reactants.
- Pressure: In reactions involving gases, increasing pressure will favor the side with fewer moles of gas.
- Catalysts: While catalysts speed up the rate at which equilibrium is reached, they do not affect the position of the equilibrium.
Chemical equilibrium is established when a reversible reaction occurs, and the rates of the forward and reverse reactions are equal. This can happen over time as reactants are converted to products and vice versa. The equilibrium state can be represented by the equilibrium constant (K), which quantifies the ratio of product concentrations to reactant concentrations at equilibrium at a given temperature.
There are several types of equilibrium, including:
- Static Equilibrium: Occurs when an object is at rest, and all forces acting on it balance out.
- Dynamic Equilibrium: Occurs when an object is in motion at a constant velocity, with net forces still equal to zero.
- Chemical Equilibrium: Refers to a reversible chemical reaction where the concentrations of reactants and products remain constant over time.
- Thermal Equilibrium: Describes the state where two objects in contact with each other reach the same temperature and no heat flows between them.
Questions related to
On Question asked by student community
Hello there
The MCQs of Ionic equilibrium are available on Career 360 website. You can access Multiple Choice questions (MCQ) along with detailed answers. There are videos, Mock tests, practice sets are also available on the website. Neverthe less by applying filter you can download MCQs of prefered year.
Hope
Hello Greetings
Chemical equilibrium can be a challenging chapter, but with some easy tips and practice, you'll become proficient in solving problems. Here are some tips to help you:
Understand the Basics
1. *Law of Mass Action*: Understand that the rate of a reaction is proportional to the product of
Hello Greetings
Equilibrium can be a challenging chapter, especially with the numerous equations involved. Here are some easy tips to help you study and solve problems in this chapter:
Understanding Equilibrium Equations
1. *Start with the basics*: Begin by understanding the fundamental concepts of equilibrium, such as the law of
Hello! Greetings from Careers360!
To achieve equilibrium for the third charge q3 placed between two fixed point charges q1 and q2, we need to consider the forces acting on q3. The sign of q3 will indeed depend on the signs of q1 and q2.
1. If q1and q2 have the
