Ethane - Definition, Properties, Preparation, Uses with FAQs
In this article, we will include how C2H6 forms ethane, ethane properties, how ethane covalent bond is formed, ethane uses, mass of ethane, what is the molecular structure of ethane, what is C2H6 ethane properties, what is the chemical formula of ethane, ethane boiling point, IUPAC naming of ethane, what is the electronic structure of ethane, what is the general formula of ethane, what is ethane molar mass, what is ethane molecular weight.
This Story also Contains
- What is Ethane?
- What is the Formula of Ethane?
- Properties of Ethane
- Bonding in Ethane
- Preparation of Ethane
- Uses of Ethane are:
What is Ethane?
When we talk about the organic compounds we come to know that on the basis of the types of bonds present in the carbon compounds we decide what their naming should be. So the simplest of all is single bonds present between the carbon atoms present in a compound. Here we are talking about ethane so we must know that as the name suggests eth in the beginning so we must know that it contains two carbon atoms bonded to each other by a single bond. ![]() is called ethane.
is called ethane.
Also read -
What is the Formula of Ethane?
The ethane formula is ![]() .
.
When we talk about the actual structure of the ethane, it is already known that the organic groups ending with the “ane” are supposed to contain the single bond. They are considered to be the simplest of all others and so is their naming. As the chemical formula suggests, in ethane 2 carbon atoms are present and 6 hydrogen atoms are present.
Ethane structure or ![]() structure of C2H6 structure:
structure of C2H6 structure:
![]() chemical name is ethane..
chemical name is ethane..
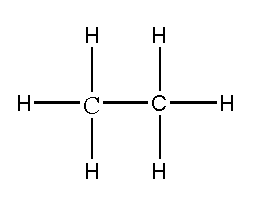
Properties of Ethane
We will discuss the properties of ethane here.
As it is known that there are two types of properties for any compound and they are physical properties and chemical properties. Physical properties include ethane colour, ethane boiling point, ethane molar mass, ethane molecular weight, ethane vapour pressure. So ethane is a colourless gas and also odourless gas. Talking about the molar mass of ethane it is 30.070g/mol. Then, the density of ethane is 1.3562kg/m3 when the gas is at 0 degree celsius. Further when the liquid is at -88.5degree celsius then density has to be 544.0kg/m3. The melting point of ethane is -182.8 degrees Celsius, -296.9 degrees Fahrenheit and 90.4 kelvin, the boiling point of ethane is -88.5 degrees Celsius, -127 degrees Fahrenheit, and 184.6 kelvin and the vapour pressure of ethane is 3.8453MPa.
Bonding in Ethane
Before heading towards the chemical properties we will first discuss the bonding or more precisely the type of bonding in the ethane. Basically, the bonding in the compound is according to the valence orbital theory. In ethane, as there are two carbons and both the carbons are bonded with tetrahedral geometry or have four bonds, therefore, it shows covalent bonding in it and that too it has seven covalent bonds – carbon has three each with the hydrogen atom and one with each other. As here we are talking about the geometry of the molecule also so it can be best determined by the electronic configuration of the ethane molecule which can be further explained below. This topic can be asked, “ explain the type of bonding in ethane”.
On the basis of the electronic configuration of carbon, there are four valence electrons in the outermost shell and there is one valence electron in the outermost shell of hydrogen so the three valence electrons of carbon get shared with the three hydrogen atoms and one with the other carbon atom.
Preparation of Ethane
Ethane can be easily prepared by methyl iodide.
![]()
When methyl iodide is reacted with the sodium in the presence of dry ether we get ethane and sodium iodide as the by-product. This reaction is known as the wurtz reaction.
Then we will discuss the preparation of ethane from sodium propionate.
![]()
When sodium propanoate reacts with soda lime ethane is obtained. Here we are talking about the soda-lime so we must know that soda lime is a mixture of NaOH and CaO.
Then comes how methyl bromide leads to the preparation of the ethane
It is the same reaction as done with the methyl iodide because the halogen group is present here which will further lead to the formation of ethane by the Wurtz reaction.
As we have discussed the properties of ethane so we must know its uses also.
Also Read:
Uses of Ethane are:
It is used for the production of ethene by steam cracking.
It can also be used as a refrigerant in cryogenic refrigerant systems.
Also, ethane is used primarily as a raw material for the production of ethylene for further reduction of plastics, detergent making, and also fruit ripening.
It is used by the scientists also in liquid form. It is used in liquid form because it vitrifies the water rich materials. It can be liquefied and then used as a fuel for automobiles.
It can also be used for the production of acetic acid, alcohol, and other similar organic compounds.
We must note here that when it is used in the absence of oxygen then it can be poisonous.
It is also used to form ethyl chloride which is used to make tetraethyl lead.
So , these are some of the basic or we can say common uses of ethane.
conclusion: as we have discussed so much about ethane then we should know its common name which is dimethyl as it has two methyl groups present.
Also in everyday life we must have listened to the ethane gas then what is it?
It is the ethane itself as we have already discussed that ethane is a colourless, odourless, and compressible gas.
We have discussed each and every point about ethane in detail.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
As there are three carbon atoms and six hydrogen atoms then there must be a double bond between any two carbon atoms so therefore it is called propene.
If three moles of ethane are present then what we need to do is that we will have 6 carbon atoms and 18 hydrogen atoms and then accordingly each figure will get altered.
Questions related to
On Question asked by student community
Correct Answer: CNG
Solution : The correct answer is CNG.
The substance described as odourless, tasteless, and non-toxic, composed of 93.05% methane, nitrogen, carbon dioxide, propane, and traces of ethane, is compressed natural gas (CNG). CNG is a cleaner-burning fuel commonly used as an alternative to traditional gasoline and
Correct Answer: Butane and Propane
Solution : The correct option is Butane and Propane.
Propane (C3H8) and Butane (C4H10) make up the majority of the two Hydrocarbon gases that make up liquefied petroleum gas (LPG). These gases are produced during the processing
