Ethanol Formula - Structure, Properties, Processing, Uses, FAQs
What is ethyl alcohol?
Ethyl alcohol or ethanol alcohol is a naturally occurring plant fermentation by product and it can also be produced through the hydration of ethylene. It consists of an ethyl group and a hydroxyl group. The common name of ethanol is spirit.
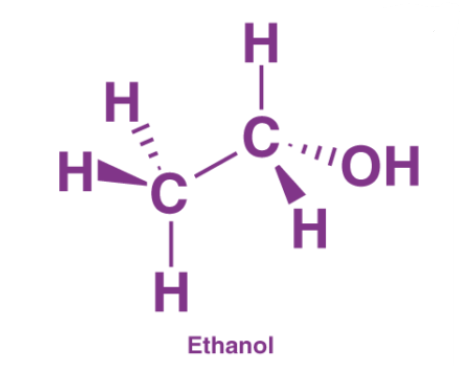
Ethanol Structural Formula
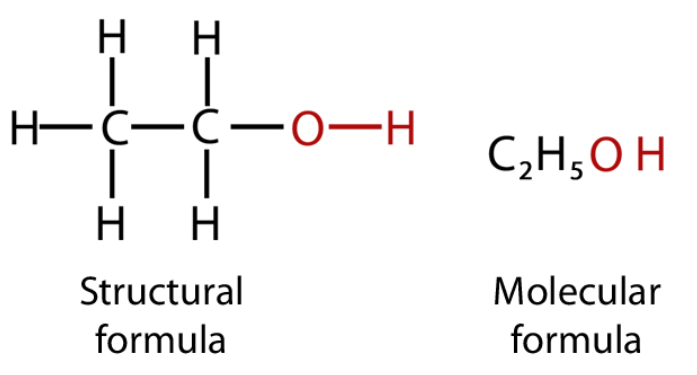
Ethanol is a primary alcohol. In the ethane unit, if one of the hydrogens is substituted by a hydroxyl group, the resulting structure will be ethanol. The structural/ molecular/ chemical formula of ethanol is: CH3CH2OH. CH3CH2OH name is ethanol.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is ethyl alcohol?
- Ethanol Structural Formula
- Drinking Alcohol Formula
- Properties
- Processing of Ethanol
- Ethanol to Ethanal
- Formation of Vinegar From Alcohol
- Uses
- Toxicity
Ethyl alcohol structure
Alcohol chemical formula
The general formula of alcohol is CnH 2n+1OH ; where n represents the number of carbon atoms in the molecule.
IUPAC name of ethyl alcohol
IUPAC name of ethyl alcohol is: Ethanol
Drinking Alcohol Formula
Ethanol and water are the main ingredients in most alcoholic beverages. In some very sweet liqueurs, the sugar content can be higher than that of ethanol content. Because of the presence of ethanol, the fermentation of carbohydrates with yeast is done. It can also be manufactured from ethylene, i.e., obtained from cracked petroleum hydrocarbons.
Hence, the molecular formula is: CH3CH2OH
Alcohol chemical name (alcohol scientific name)
The chemical or scientific name of alcohol is Ethanol.
Properties
Ethanol is usually a clear colourless liquid with a specific vinous odour and pungent taste.
Highly volatile
flammable
Boiling point 78.5˚C.
Density 6.5 lb / gal.
Vapours are heavier than air.
Processing of Ethanol
Manufacture of ethanol can be done in two main processes :
the fermentation of carbohydrates in presence of anaerobic bacteria (the method used for alcoholic beverages) and
the hydration of ethylene.
carbohydrates (glucose) are Fermented in presence of anaerobic bacteria, such as, yeast; to produce ethanol. The raw materials utilized for the preparation of industrial alcohol are sugar crops including beets and sugarcane and grain crops, such as corn (maize).
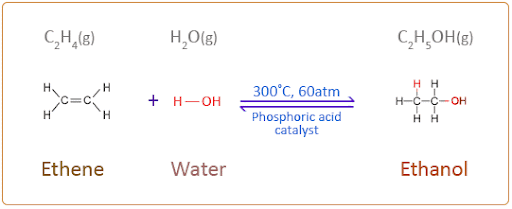
Hydration of ethylene is carried out by passing a mixture of ethylene and a large excess of steam (H2O) at high temperature (~300˚C) and pressure (~ 60atm) over an acidic catalyst, such as a phosphoric acid catalyst.
Purification
Ethanol produced by the above methods is obtained as a dilute aqueous solution that is concentrated by fractional distillation. By direct distillation best the constant-boiling-point mixture of ethanol containing 95.6% by weight of ethanol can be obtained as yield. Dehydration of that constant-boiling-point mixture yields anhydrous, or absolute alcohol.
The absolute alcohol formula C2H5OH
Ethanol molecular weight:
46.07 gm.mol-1
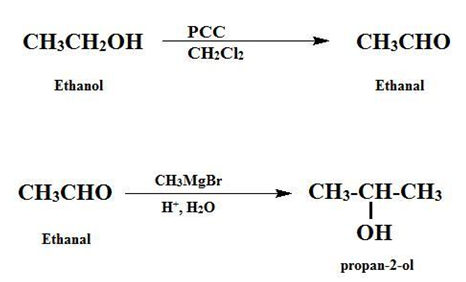
Ethanol to Ethanal
During conversion (oxidation) of ethanol to ethanal, a molecule of hydrogen is removed and a C-O bond is converted to a C=O bond. During this reaction, the hydroxyl group of alcohol gets converted to the carbonyl group of aldehyde. If further oxidation occurs, ethanal is converted to ethanoic acid.
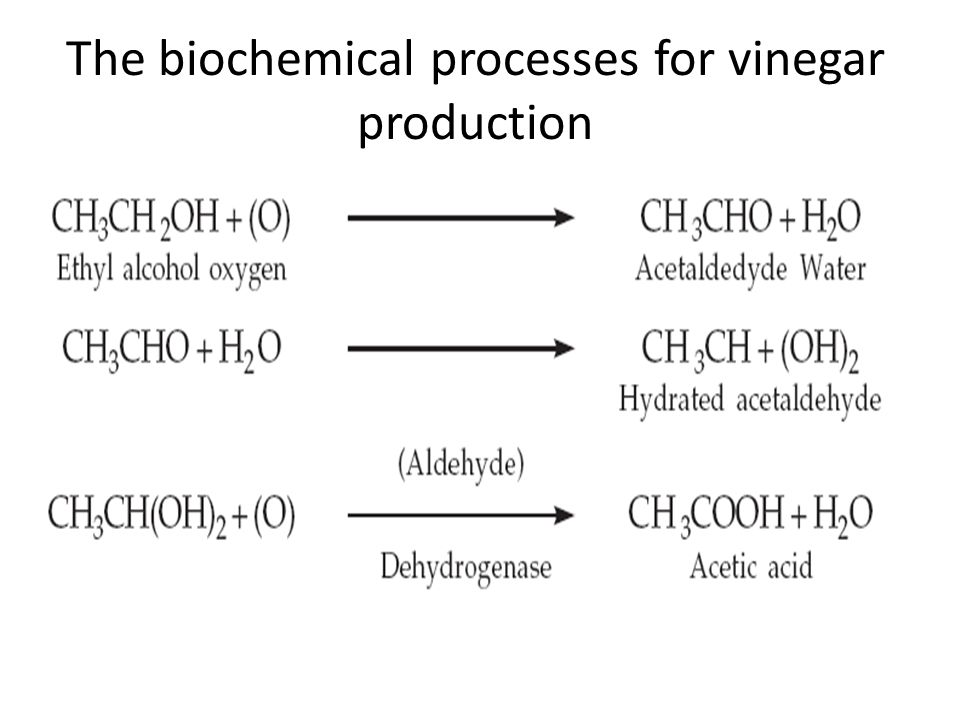
Formation of Vinegar From Alcohol
The formation of vinegar from alcohol is caused by Anaerobic bacteria such as acetic acid bacteria.
The vinegar is formed when acetic acid bacteria acts on alcoholic beverages such as wine. Those bacteria are used to perform specific oxidation reactions through processes called “oxidative fermentations” which produce vinegar as a by-product.
Uses
Ethanol plays a vital role as an antiseptic drug, a polar solvent in chemical synthesis, a depressant for a central nervous system, a neurotoxin, a disinfectant, a teratogenic agent, and an Escherichia coli metabolite.
Ethanol is used in alcoholic beverages in proper dilutions. Ethanol is also utilised as a reagent in chromatography and in organic chemistry. It is also used as an industrial solvent.
It is used to manufacture denatured alcohol, pharmaceuticals (rubbing compounds, lotions, tonics, colognes), perfumery, and as an octane booster in gasoline.
Ethanol is a very good central nervous system (CNS) depressant that enhances the inhibitory effects of GABA at the GABA-A receptor, GABA stands for gamma-aminobutyric acid.
It stimulates the release of dopamine and serotonin.
Consuming a moderate amount of ethanol reduces myocardial infarction risk.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 11 Alcohols, Phenols and Ethers
- NCERT notes Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
Toxicity
Ethanol toxicosis symptoms are vomiting, lethargy, ataxia, and recumbency. In severe cases, hypothermia, tremors, acidosis, diarrhea, seizures, disorientation, vocalization, hypotension, tremors, tachycardia, coma, can lead to death may occur. Alcohol is directly irritating to the stomach which causes vomiting.
High alcohol consumption increases the concentration of ethanol in blood levels and also stimulates emesis. During intoxication, vomiting occurs due to high blood ethanol concentrations. As a result, the muscle gets paralyzed which increases the risk for aspiration.
Intoxication of ethanol reduces peripheral oxygen delivery and also causes mitochondrial oxidative dysfunction which results in hypoxia.
Ethanol toxicity causes hypothermia, which leads to a lowered body temperature
High ethanol consumption is associated with the risk of cancer mortality in women.
Beer consumption by lactating women may temporarily impair the motor function of nursing infants.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The formula of ethyl is CH3CH3
The mass percent of oxygen in ethanol is 34.73%
Molecular formula of hexanol: C₆H₁₄O
Also Read
30 Sep'24 11:38 AM
30 Sep'24 08:59 AM
21 Jul'22 03:44 PM
16 Jul'22 05:44 PM
16 Jul'22 05:33 PM
16 Jul'22 05:08 PM
16 Jul'22 05:01 PM