Fajan’s Rule
Fajans' Rule (Partial Covalent Character of Ionic Bonds) is one of the most important concepts, simply because it tells a lot about the nature of the chemical bond, more precisely about the transition from ionic to covalent bonds. Presented by Polish chemist Kazimierz Fajans in 1923, this rule has been of much help in presenting a systematic approach toward the factors that impact the characteristics of bonds formed between atoms. The essential postulate of Fajans' Rule is that the degree of covalency in what is normally considered to be an ionic bond can vary enormously depending on the size and charge of the ions involved.
This Story also Contains
- Fajans' Rule
- Characteristics of Covalent Compounds
- Some Solved Examples
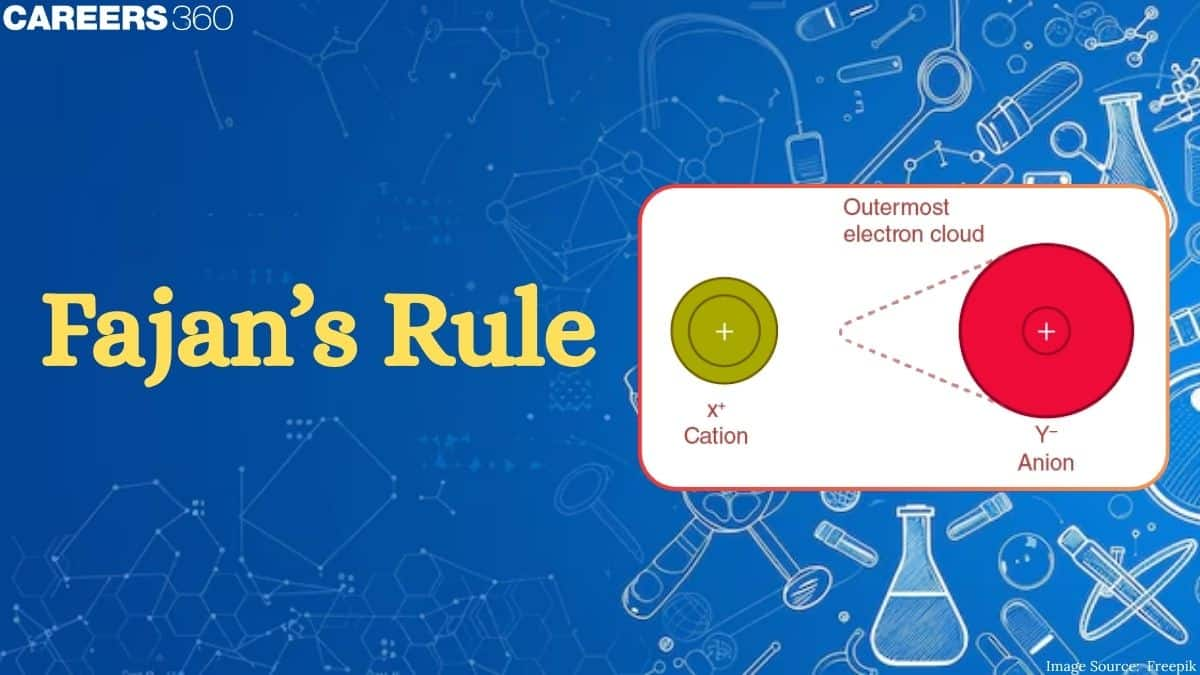
Although in an ionic compound, the bond is considered to be 100% ionic, actually it has some covalent character. Thus, just as covalent bond has some ionic character, ionic bonds have some covalent character. This was explained by Fajan.
In this article, we will cover the concept of Fajans' Rule. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read:
Fajans' Rule
The covalent character in ionic bonds is determined by Fajan’s rule. It simply says that no ionic bond is completely ionic, there is always some covalent character in ionic bond. When a cation approaches an anion, then the electron cloud of the anion is distorted and shifted towards the cation, this distortion is known as the polarisation of the anion.
The ability of the cation to distort the anion is known as polarising power and the ability of the anion to get distorted is known as polarisability.
.jpg)
Also Check:
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
The covalent character in ionic bonds depends on the following factors:
- Size of the cation: The smaller the size of the cation, the larger will be its polarisability.
- Size of the anion: The larger the size of the anion, the larger will be its polarisability.
- Charge on cation and anion: The more the charge on a cation more polarising power. Further, the more the charge on an anion, the more will be its polarisability.
- Electronic configuration of the cation: If two cations have the same size and charge, then the one with pseudo noble gas configuration (with 18 electrons in the outermost shell) has greater polarising power than the other with noble gas configuration (with 8 electrons in the outermost shell). This explains why $\mathrm{Cu}^{+} \mathrm{Cl}^{-}$ is more covalent than $\mathrm{Na}^{+} \mathrm{Cl}^{-}$.
Thus covalent character for chlorides follows this order:
$\mathrm{NaCl}<\mathrm{MgCl}_2<\mathrm{AlCl}_3$
In this case, the charge on the cation increases, thus its polarising power also increases.
Further, for cation size, the covalent character follows the below order: $\mathrm{LiCl}>\mathrm{NaCl}>\mathrm{KCl}>\mathrm{CsCl}$
In this case, as the size of the cation increases, its polarising power decreases.
Also Read:
Characteristics of Covalent Compounds
- Physical State: Unlike ionic compounds (which generally exist as solids), the covalent compounds exist in all the three states, viz., solid, liquid and gaseous.
- Solubility: Covalent compounds are generally soluble in organic (non-polar or weakly polar) solvents but insoluble in water and other polar solvents.
- Electrical conductivity: Since there are no free ions in covalent compounds to conduct electricity, they are bad conductors of electricity.
- Melting and Boiling points: Covalent compounds have low melting and boiling points because the molecules in covalent compounds are held together less rigidly (by weak van der Waals forces), than in case of ionic compounds.
Related Topics:
Some Solved Examples
Example 1:
Which one is the least ionic in the following compounds?
1) AgCl
2) KCl
3) NaCl
4) CsCl
Solution: The covalent character in ionic bonds is greater when the size of the cation is smaller and the charge on the cation is greater. Since Ag+ has a pseudo-noble gas configuration, AgCl will have the greatest covalent character.
Hence, the answer is option (1) AgCl.
Example 2:
Arrange the following in decreasing order of covalent character: LiCl, KCl, NaCl, RbCl.
1) KCl > LiCl > NaCl > RbCl
2) NaCl > KCl > LiCl > RbCl
3) LiCl > NaCl > KCl > RbCl
4) RbCl > NaCl > KCl > LiCl
Solution: The more the polarisation, the more the covalent character. As the size of the cation increases, the ability of the cation to distort the electron cloud of the anion decreases, resulting in a decrease in polarisation and covalent character. Therefore, the order is LiCl > NaCl > KCl > RbCl.
Hence, the answer is option (3).
Example 3:
Polarisability of halide ions increases in the order:
1) $\mathrm{F}^{-}, \mathrm{I}^{-}, \mathrm{Br}^{-}, \mathrm{Cl}^{-}$
2) $\mathrm{Cl}^{-}, \mathrm{Br}^{-}, \mathrm{I}^{-}, \mathrm{F}^{-}$
3) $\mathrm{I}^{-}, \mathrm{Br}^{-}, \mathrm{Cl}^{-}, \mathrm{F}^{-}$
4) $\mathrm{F}^{-}, \mathrm{Cl}^{-}, \mathrm{Br}^{-}, \mathrm{I}^{-}$
Solution: The polarisability of any anion is dependent on its size and charge. The greater the size, the greater the polarisability. Therefore, the correct order is $\mathrm{F}^{-} \mathrm{Cl}^{-}, \mathrm{Br}^{-}, \mathrm{I}^{-}$.. Hence, the answer is option (4).
Example 4:
Arrange the following in the decreasing order of their covalent character: (A) LiCl, (B) NaCl, (C) KCl, (D) CsCl.
1) (A) > (C) > (B) > (D)
2) (B) > (A) > (C) > (D)
3) (A) > (B) > (C) > (D)
4) (A) > (B) > (D) > (C)
Solution: Covalent character increases with the increase in charge density of the cation. Therefore, the order of covalent characters is LiCl > NaCl > KCl > RbCl.
Hence, the answer is option (3).
Example 5:
The number of following factors which affect the percent covalent character of the ionic bond is_____
[JEE Main 2023]
(1) Polarising power of cation
(2) Extent of distortion of anion
(3) Polarisability of the anion
(4) Polarising power of anion
Solution:
The three factors in Fajan's rules:
- The polarising power of the cation
- The polarisability of the anion
- The extent of distortion of the anion.
These factors are responsible for the covalent character in an ionic bond.
-
Polarising power of the cation:
A smaller cation with a higher positive charge has a greater ability to distort the electron cloud of the anion, leading to more covalent character.
-
Polarisability of the anion:
A larger anion with a more diffuse electron cloud is more easily distorted by the cation, increasing the covalent character.
-
Extent of distortion of the anion:
This is the result of the first two factors; the greater the distortion, the more the electron density is shared between the ions, and the more covalent the bond is.
Hence, the answer is 3.
Practice More Questions From the Link Given Below:
Summary
In other words, Fajans' Rule is one of the important theories explaining the nature of chemical bonds, mainly with respect to the transition of ionic to covalent character. It was based on factors affecting polarizing power and polarizability dependent upon the size and charge of cations and anions. Coupled with this rule, there are three main postulates that provide a guideline for assessing the covalency character of ionic bonds.
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
It is especially useful when comparing salts having similar formulas but different ionic sizes or charges, or in “borderline” cases where the bond is neither fully ionic nor fully covalent.
Though Ag⁺ and K⁺ may have similar ionic radii, Ag⁺ has higher polarizing power because of electronic structure (pseudo inert gas configuration), so it distorts Cl⁻ more, enhancing covalent character in AgCl.
Charge generally has stronger influence than small variations in size, but when cations have the same charge and size, electronic configuration (e.g., pseudo‑noble gas) becomes decisive.
NaCl is more covalent than KCl because the Na⁺ ion is smaller and can polarize Cl⁻ more strongly than K⁺ can.
If a cation has a pseudo‑noble gas configuration (for example, involving filled d‑orbitals), it can polarize the anion more effectively than a cation with a simple ns² np⁶ configuration, all else being equal.
Fajan’s rule states that an ionic bond will show more covalent character (i.e. more sharing of electron density) when the cation is small and highly charged (strong polarizing power) and the anion is large and easily polarizable.
If a cation has a pseudo‑noble gas configuration (for example, involving filled d‑orbitals), it can polarize the anion more effectively than a cation with a simple ns² np⁶ configuration, all else being equal.
NaCl is more covalent than KCl because the Na⁺ ion is smaller and can polarize Cl⁻ more strongly than K⁺ can.
Charge generally has stronger influence than small variations in size, but when cations have the same charge and size, electronic configuration (e.g., pseudo‑noble gas) becomes decisive.
Considering only cation size or only charge, ignoring the combined effect
Forgetting to consider anion polarizability
Ignoring the role of electronic configuration or pseudo‑noble gas effects
Applying the rule where polarization is negligible (very ionic compounds)