Frequency, Time Period And Angular Frequency
Chemistry in other words principally focuses on electron actions in an atom. With the help of Niels Bohr's atomic model, in which electrons were envisaged to revolve in circular orbits centred at the nucleus with fixed energy, it has provided a great advancement in understanding the atomic structure and chemical characteristics. Each specified by a principal quantum number is an energy state. Further, we get interested in the time period and frequency of an electron’s revolution in these orbits. Where, ‘time-period, ‘ varies as is the time taken by an electron to complete one full orbit around the nucleus, and ‘frequency, ‘, is the number of complete orbits per unit of time. These are important because they affect the energy state of electrons and the witnessed results during atomic emission and atomic absorption spectroscopy. Through these studies, chemists learn about transitions that occur to electrons, the energy that is absorbed or emitted, and an electronic configuration that determines the stability and reactivity of the atom.
This Story also Contains
- Time Period and Frequency of Revolution of an Electron in the nth Bohr orbit
- Solved Examples Based on Time Period and Frequency of Revolution of an Electron in the nth Bohr Orbit
- Practice More Questions From the Link Given Below:
- Conclusion
In this article, we will cover the concept of Time Period and Frequency of the Revolution of an Electron in the nth Bohr orbit. This concept falls under the broader category of Atomic structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Let us study in detail the time Period and Frequency of Revolution of an Electron in the nth Bohr orbit to gain insights into this topic and solve a few related problems.
Time Period and Frequency of Revolution of an Electron in the nth Bohr orbit
As the spectroscopists, the time-period and frequency of an electron’s revolution in the nth Bohr orbit are basic necessary parameters in chemistry. Niels Bohr’s atomic model introduced the concept of orbits in which electrons revolve in circles around the nucleus and each orbit is defined by a number called the principal quantum number (n). The time-period (Tn) is the time taken by a particular electron to revolve in a particular orbit and can be determined as:
($T_n=\frac{2 \pi r_n}{v_n}$), where ($r_n$) is the radius of the nth orbit and ($v_n$.) is the velocity of the electron in that orbit
On the other hand, frequency [fn] is defined as the number of vibrations per second or the number of complete oscillations per second, it is connected with the difference in energy levels between the concerned orbits. These concepts are very vital in spectroscopy, whereby they determine the emission and absorption of photons which correspond to energy transitions. They also determine the stability of the atomic nucleus as well as the chemical activity, giving details on the status of elements and molecules.
Although the precise equations for the time period and frequency of revolution are not required, it is still a good idea to look at the variations of these with the atomic number (Z) and the orbit number (n).
We know that Time period (T) is the time required for one complete revolution and that Frequency (f) is the inverse of the time period.
The time period (T) of an electron in the nth Bohr orbit is proportional to $\mathrm{n}^3 / \mathrm{Z}^2$ (where n is the principal quantum number and Z is the atomic number), and the frequency (f) of revolution is proportional to $Z^2 / n^3$. This means the time period increases with the cube of the principal quantum number, while the frequency decreases with the cube of the principal quantum number.
$\begin{gathered}\therefore T=\frac{\text { distance }}{\text { time }}=\frac{2 \pi r}{v} \\ \because \mathrm{r} \propto \frac{\mathrm{n}^2}{\mathrm{Z}} \text { andv } \propto \frac{\mathrm{Z}}{\mathrm{n}} \\ \therefore T \propto\left(\frac{n^2}{Z} \times \frac{n}{Z}\right) \propto\left(\frac{n^3}{Z^2}\right) \\ \therefore v=\left(\frac{1}{T}\right) \propto\left(\frac{Z^2}{n^3}\right)\end{gathered}$
You must remember all the above formulas and relations.
Also Read:
For a better understanding of the topic and to learn more about Frequency, Time Period And Angular Frequency with video lesson, the link has been provided below:
Recommended YouTube video (Frequency, Time Period And Angular Frequency):
Solved Examples Based on Time Period and Frequency of Revolution of an Electron in the nth Bohr Orbit
Example 1: Ratio of frequency of revolution of the electron in the 2nd excited state of He+and 2nd state state of hydrogen is.
1) $\frac{32}{27}$
2) $\frac{27}{32}$
3) $\frac{1}{54}$
4)$\frac{27}{2}$
Solution:
$
\begin{aligned}
&\begin{gathered}
\frac{f_1}{f_2}=\frac{z_1^2}{n_1^3} \times \frac{n_2^3}{z_2^2} \\
\Rightarrow n_1=3, n_2=3, z_1=2, z_2=1
\end{gathered}\\
&\therefore \text { putting these value in the equation we get }\\
&\frac{2^2}{3^3} \times \frac{2^3}{1}=\frac{32}{27}
\end{aligned}
$
Hence, the answer is the option (1).
Example 2: The time taken for an electron to complete one revolution in the Bohr orbit of the hydrogen atom is
1) $\frac{4 \pi^2 m r^2}{n h}$
2) $\frac{n h}{4 \pi^2 m r}$
3) $\frac{n h}{4 \pi^2 m r^2}$
4) $\frac{h}{4 \pi m r}$
Solution:
We know that time period is the time taken for one complete revolution.
$T=\frac{2 \pi r}{V}$(1)
Now, we know that the angular momentum of an electron in $n^{\text {th }}$ orbit is given by $m V r=\frac{n h}{2 \pi} \Rightarrow V=\frac{n h}{2 \pi m r} \ldots \ldots$
From equations (1) and (2),
$
\therefore T=\frac{2 \pi r}{V}=\frac{2 \pi r}{\frac{n h}{2 \pi m r}}=\frac{4 \pi^2 m r^2}{n h}
$
Hence, the answer is the option (1).
Example 3:Determine the frequency of revolution of the electron in 3rd Bohr's orbit in hydrogen atom:
1) 0.024 x 1016Hz
2) 3.4 x 1016Hz
3) 8.13 x 1016Hz
4) 0.054 x 1016Hz
Solution:
As we have learnt,
Frequency = 1/(Time period)
Period = Total distance covered/velocity
= 2πr/v
Thus, frequency = v/2πr
Now, velocity of electron in 3rd Bohr’s orbit = 2.16 x 106(1/3)m/s
= 0.72 x 106m/s
And, radius of 3rd Bohr’s orbit $=0.53 \times 10^{-10}\left(3^2 / 1\right)$
$=4.77 \times 10^{-10} \mathrm{~m}$
Now, frequency = $=\mathrm{v} / 2 \pi \mathrm{r}$
$=0.72 \times 10^6 \mathrm{~m} / \mathrm{s} /\left(2 \times 3.14 \times 4.77 \times 10^{-10} \mathrm{~m}\right)$
$=0.024 \times 10^{16} \mathrm{~Hz}$
Hence, the answer is the option (1).
Example 4: The ratio of the orbital frequency of electron of hydrogen in the 3rd and 2nd orbital is:
1) 3.37
2) 0.29
3) 0.44
4) 2.25
Solution
We know that,
Frequency $\propto \frac{\mathrm{Z}^2}{\mathrm{n}^3}$
For the same value of $Z$,
$
\begin{gathered}
f \propto \frac{1}{n^3} \\
\frac{f_3}{f_2}=\frac{\frac{1}{3^3}}{\frac{1}{2^3}}=\frac{8}{27}=0.29
\end{gathered}
$
Hence, the answer is the option (2).
Example 5:
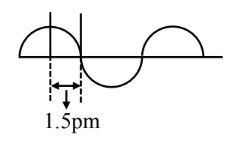
A hypothetical electromagnetic wave is show below.
The frequency of the wave is $\mathrm{x} \times 10^{19} \mathrm{~Hz}$. $x=$ $\qquad$ (nearest integer).
[JEE Main 2024]
Solution:
$\begin{aligned} \lambda & =1.5 \times 4 \mathrm{pm} \\ & =6 \times 10^{-12} \text { meter } \\ \lambda v & =\mathrm{C} \\ 6 & \times 10^{-12} \times v=3 \times 10^8 \\ v & =5 \times 10^{19} \mathrm{~Hz}\end{aligned}$
Hence, the answer is (5).
Practice More Questions From the Link Given Below:
Conclusion
Finally, It is comprehensible and quite reasonable that two of the most important concepts in atomic structure and atomic behaviour are the time period and frequency of revolution of the electrons in Bohr orbits. Niels Bohr’s model was essential in giving a foundation for a concept which stated that electrons orbit in a particular path with fixed energies. Regarding these orbits, two quantitative parameters known as the time period and the frequency affect atomic spectra, which show various lines for energy transitions. These parameters are basic in spectroscopy where the kinds of elements and composition of chemicals by emitted or absorbed radiation are determined. Also, they stress atomic stability, asserting that transitions between energy levels determine chemical activity and bonding characteristics. In addition to theoretical significance, knowledge about electron orbits improves technological advancements, like lasers and quantum computing, based on specific energy changes. Hence one can deduce an understanding of time-period and frequency concerning Bohr orbits that enhances understanding of atomic physics and stimulates improvement in fields of science as well as technology.
Frequently Asked Questions (FAQs)
In periodic motion or oscillations, the time period T is the time taken for one complete cycle of motion (one oscillation). The angular frequency ω is a measure of how fast that motion goes “in terms of angle” — how many radians are swept per unit time.
Frequency, usually denoted by fff, is the number of complete cycles (or oscillations) that occur per unit time — its unit is Hertz (Hz), which means cycles per second. Angular frequency, denoted by ω, instead measures how fast the phase of a wave or oscillation changes, in radians per second. Because one complete cycle corresponds to an angular change of 2π radians, angular frequency is related to ordinary frequency by ω=2πf. Thus for a given oscillation, frequency tells you how many cycles per second you get, while angular frequency tells you how many radians of “angle” (phase) the system sweeps per second.
Time period (symbol: T) is the duration of time it takes for one complete cycle of a repeating or periodic motion. For example, if something oscillates (moves back and forth), the period is the time it takes to go through one full oscillation and return to its original state. Its unit is seconds (s).
Frequency (symbol: fff) is the number of cycles (or oscillations) that occur in one unit of time. It tells how often the repetition happens per second. The unit of frequency is Hertz (Hz), which is “cycles per second.”
The time-period (Tn) is the time that takes an electron to go around the nucleus in the particular orbit it is in.
Frequency (νn) is given by the number of complete orbits an electron has per unit of time in its nth Bohr orbit, the parameter that characterizes the electron's angular momentum and energy.
They define the change-over of energy between two orbits of electrons and thus decide the release or absorption of photons resulting in the production of spectral lines engaged in the identification of elements in chemical analysis.