General Principles and Processes of Isolation of Elements - Notes, Topics, Formula, Books, FAQs
This chapter deals with the processes and isolation of metals from their sources. Metals are always obtained from their respective ores. These ores are found in the earth's crust and they are obtained by mining. These ores contain some form of impurities known as gangue. Thus, metals are obtained in their purified form by following several steps.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important Topics General principles and processes of Isolation of Elements
- Overview Of The General Principle And Process Of Isolation Of Metals
- Uses Of Aluminium, Copper, Zinc And Iron
- How To Prepare For General Principle And Process Of Isolation Of Metals?
- Prescribed Books For General Principle And Process Of Isolation Of Metals

Majorly, the general principles and processes of isolation of elements from their ores follow the given steps:
(i) Concentration of the ore
(ii) Isolation of the metal from its ore
(iii) Purification of the metal
This chapter is all about the extraction and purification of metals. Various types of methods and processes specified for particular metals are described in this chapter. It is a very important chapter as it teaches you how these metals you use in your daily life are purified. From the practical and real-life applications point of view if we talk about then these metals are highly important for all of us.
In this article, you will get complete information about this chapter. This article will also help you regarding how to prepare for this chapter and the best-described books for preparation.
Important Topics General principles and processes of Isolation of Elements
Metallurgy:
Metallurgy is the science and technology concerned with the properties of metals and their production and purification. It deals with the study of the physical and chemical behavior of metallic elements, their intermetallic materials, and alloys.
Froth Floatation Process:
Consider the separation of the worthless rock as a way to save valuable minerals from churning out raw materials for the modern world. All this is a complicated method to go about; it is called the Froth Floatation Process, the extraction of minerals such as copper, lead, and zinc from ores.
Leaching Process:
Leaching can be defined as the process of extracting a given soluble substance by washing or dissolving it with a liquid solvent. In the activity of metallurgy, disengagement in an aqueous solution is used for the extraction of metals from mineral materials.
Cyanide Process:
The cyanide process, also called the Macarthur-Forrest Cyanide Process, method of extracting silver and gold from their ores by dissolving them in a dilute solution of sodium cyanide or potassium cyanide. The process was invented in 1887 by the Scottish chemists John S. MacArthur, Robert W. Forrest, and William Forrest.
Calcination And Roasting:
The calcination and roasting processes in the conversion of ore into its oxide. Calcination involves the process of heating ore without air to remove the volatile substances, whereas roasting is termed a process by which ore is heated in excess oxygen to enhance the removal of sulfur and other impurities.
Flux And Slag:
Flux and slag are words that apply to metallurgy. Flux is a chemical substance that combines with the impurities present in the roasted or calcined ore to form an easily fusible material. Slug is the fusible material formed by the reaction of flux and gangue.
Refining Process Against Impurities:
Metal refining is the process of purification of metal obtained from its ore and gained with impurities, aimed at increasing the purity for it to be suitable for various uses. Crucial refining processes make sure that metals regulate the required standards of different industries, technology, and items of consumer use. The method involves the removal of impurities, which cause variation in properties plus the performances of metals.
Ellingham Diagram:
The ellingham diagram is important for metallurgists to understand the thermodynamic functionality of reduction of metal oxides. It helps in the prediction of the temperatures needed when reducing metal oxides to pure metals
Extraction Of Metals: Ores And Minerals:
Metal extraction is a process of obtaining metals from their ores. The latter are defined as rocks or minerals containing enough metal to be feasibly extracted. Metal ores are first obtained from the earth's crust through mining. Softer processes are then involved in the isolation and purification of the desired metal from the ores.
Overview Of The General Principle And Process Of Isolation Of Metals
In this chapter, there are various important topics as given below that you must understand carefully. Basically, this chapter gives you knowledge of the occurrence of various elements and how they get extracted through various procedures. First, the ore needs to be concentrated with some processes like hydraulic washing, magnetic separation, etc. then finally the isolation of pure metal is done.
Concentration Of Ores
This is the process of isolation of ore from the gangue. This involves various processes that depend on the properties of the metal ore and the gangue. There are the following available methods that we use for the concentration of ore.
(i) Hydraulic Washing: This method is based on the gravity differences of the ore and the gangue. In this process, an upward stream of running water is used. The lighter gangue particles are washed away with water and the heavier ore particles get suspended on the surface.
(ii) Magnetic Separation: In this method, the ore and the gangue are separated on the basis of their magnetic properties. Either the ore or the gangue is capable of being attracted by the magnetic field and thus they able to be get separated from each other as shown in the figure.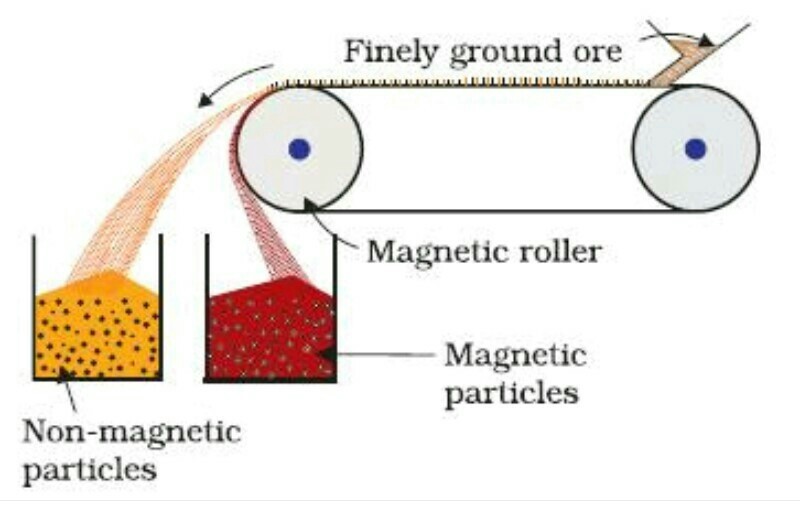
(iii) Froth Floatation Method: In this method, gangue is separated from the sulfide ores. Water is used to make a suspension of powdered ore. Then oil is used to wet the mineral ore and water is used to wet the gangue. Now a shaft is rotated at the bottom which creates forth and this froth takes the mineral with it which thus separated later.
(iv) Leaching: This process is used when the ore is soluble in some solvent. The leaching of alumina from bauxite is one of the major examples of leaching. In this process, the concentration of bauxite ore is done by digesting it with a solution of NaOH at high temperature and pressure. Thus in this way, Al2
Extraction of crude metal from concentrated ore: In this process, the concentrated ores are further converted into a form that is suitable for reduction. Thus complete isolation of metal from concentrated ore is done in two steps:
(i) Conversion of concentrated ore into the oxide
(ii) Reduction of oxide to its metal.
Now, this conversion of concentrated ore to its oxide is done in two different ways:
(i) Calcination: In this process, the ore is heated due to which the unwanted matter leaves and we are only left with the metal oxide. This process is illustrated with the following reactions:
(ii) Roasting: In this method, the ore is heated below the melting point of the metal and thus forms the metal oxide. This is illustrated in the following reactions.
(b) Reduction Of Oxide To The Metal: In this method, metal oxides are heated in the presence of a suitable reducing agent to give the pure metal. this process occurs according to the following reaction.
We use Gibbs energy to predict which must be the best-reducing agent for this process.
Refining:
After extracting the metal from the various methods as discussed above, it is still contaminated with some impurities. To remove these impurities and obtain a metal of high purity, we use various techniques as follows:
(i) Distillation: Distillation is the method is particularly important for metals like zinc and mercury. In this method, the metals are boiled and the impure metal is evaporated and the pure metal is obtained.
(ii) Liquation: This technique is specifically useful for tin and other low-melting metals. In this method, low-melting metal moves on the sloping hearth and high-melting impurities do not move, in this way, the metal is purified.
(iii) Electrolytic Refining: In this method, the principle of electrolysis is used. The impure metal acts as an anode and a strip of pure metal is used as a cathode. The electrolytic solution of the soluble salt is also of the same metal. For eg., copper and zinc are purified in this way.
(iv) Zone Refining: In this method, a mobile heater is fixed at one end of the impure metal rod. The molten zone moves along with the heater. In this way, pure metal gets crystallized and the impurities pass into the adjacent molten zone. This process is repeated several times. Germanium, silicon, etc. are purified in this way.
(v) Vapour Phase Refining: This process is done in two steps, first, the metal is converted into its volatile compound and then this volatile compound is decomposed to give pure metal. There are two important techniques i.e, "Mond Process for Refining Nickel" and "van Arkel Method for Refining Zirconium or Titanium" forthe purification of zinc and zirconium respectively.
(vi) Chromatographic Methods: In this method, a column is used as an adsorbent which has the property to adsorb the different components. The mixture of various components is passed through this column. As this mixture pass through the column, its different components adsorb at the different levels of the column. Later another solvent is used as an eluant to remove these components and purified.
Uses Of Aluminium, Copper, Zinc And Iron
- Aluminum is used for a variety of purposes like wrappers of chocolates, electricity wires, paint, and in the extraction of chromium and manganese.
- Copper is used for making electrical wires, steam pipes, and utensils. It is also used to make alloys like brass and bronze.
- Zinc is used in batteries. It is used as a reducing agent in dye-stuffs and paints. It is also used for galvanizing iron.
- Iron is used for various purposes in the manufacture of steel, toys, pipes, utensils, etc. Wrought iron is used in making of wires, bolts, chains, etc.
Some Of The Applications Of Metallurgy
- Metals like iron and steel are widely used in the construction of homes and buildings. They are hard metals and have the strength to withstand the heavyweights of these buildings and houses. They are commonly used in pillars, grilling, fencing, etc.

- In jewellery, metals like gold, silver, and platinum are used widely to make these ornaments.

In farming as well, there are many tools that they use for farming. Most of this equipment are made of iron and steel, etc.

How To Prepare For General Principle And Process Of Isolation Of Metals?
This chapter is a part of inorganic chemistry. It is completely theory-based and very easy to learn. There are only processes for the separation of metals that only you need to learn.
Most importantly, the diagrams are very important. Practice each process with the diagram, this would help a lot in board exams
This chapter is very different from every other chapter of the complete chemistry syllabus, so it does not require any pre-requisite for its preparation.
Rest this complete chapter is very simple, just be regular and be consistent in your numerical practice.
Prescribed Books For General Principle And Process Of Isolation Of Metals
First, you must finish the class XI textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow O.P. Tandon or Solomons and Fryhle. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles, and mock tests.
Frequently Asked Questions (FAQs)
The general principle of the isolation of metals involves separating metals from their ores through various processes that can include physical and chemical methods. This is typically done by using techniques such as crushing, grinding, concentration, reduction, and refining. The primary goals are to produce a pure metal and to separate it from impurities and other elements present in the ore.
An ore is a naturally occurring rock or mineral from which a valuable metal can be extracted. The process of isolation of metals begins with the mining of ores, which contain metal compounds or minerals. The metal is then separated from its ore through various processes to yield the desired pure metal.
Reduction is a chemical process where metal oxides or other compounds are converted into pure metals by removing oxygen or other elements. This process is essential in metal isolation as it transforms the metal compound (e.g., an oxide or sulfide) into its elemental form. Common reducing agents include carbon (coke), hydrogen, and various metals that are more reactive.
The two general methods of reduction are:
- Smelting: A thermal process that involves heating the ore along with a reducing agent in a furnace to extract the metal. It often requires high temperatures and results in the production of molten metal and slag.
- Electrolysis: An electrochemical process where an electric current is passed through an electrolyte solution containing the metal ions, causing the metal to deposit on the cathode. This is commonly used for metals such as aluminum and copper.
Also Read
06 Feb'25 11:59 PM
06 Feb'25 11:54 PM
09 Dec'24 11:00 AM
21 Oct'24 04:24 PM
07 Oct'24 02:35 PM
07 Oct'24 02:24 PM
07 Oct'24 02:17 PM
07 Oct'24 12:55 PM