Haloalkanes and Haloarenes - Notes, Topics, Formula, Books, FAQs
Haloalkanes and haloarenes are the class of organic compounds in which one or more than one hydrogen atom is replaced by halogen atoms. Haloalkanes are those compounds in which halogen group is attached to an aliphatic chain and haloarenes are the compounds in which halogen group is attached to an aromatic ring.
- Important Topics - Organic compounds Containing Halogens
- Overview of the Chapter
- Haloalkanes and haloarenes can be classified mainly on the basis of two aspects:
- Physical properties
- Chemical Reactions
- Applications of haloalkanes and haloarenes
- How to Prepare for Hydrocarbons
- Prescribed Books
Important Topics - Organic compounds Containing Halogens
Alkyl Halides
Alkyl halides are organic compounds containing a halogen atom (F, Cl, Br, or I) bonded to an alkyl group. Alkyl halides are classified as primary, secondary, or tertiary based on the carbon atom attached to the halogen. Alkyl halides are versatile intermediates in organic synthesis, used in the production of polymers, refrigerants, and agrochemicals.
Physical & Chemical Properties of Haloalkanes
Haloalkanes exhibit unique physical properties like higher boiling points compared to alkanes due to the polar C-X bond and their larger molecular mass. Chemically, they undergo nucleophilic substitution (SN1 and SN2 mechanisms) and elimination reactions. The reactivity depends on the halogen and the carbon skeleton.
Haloarenes
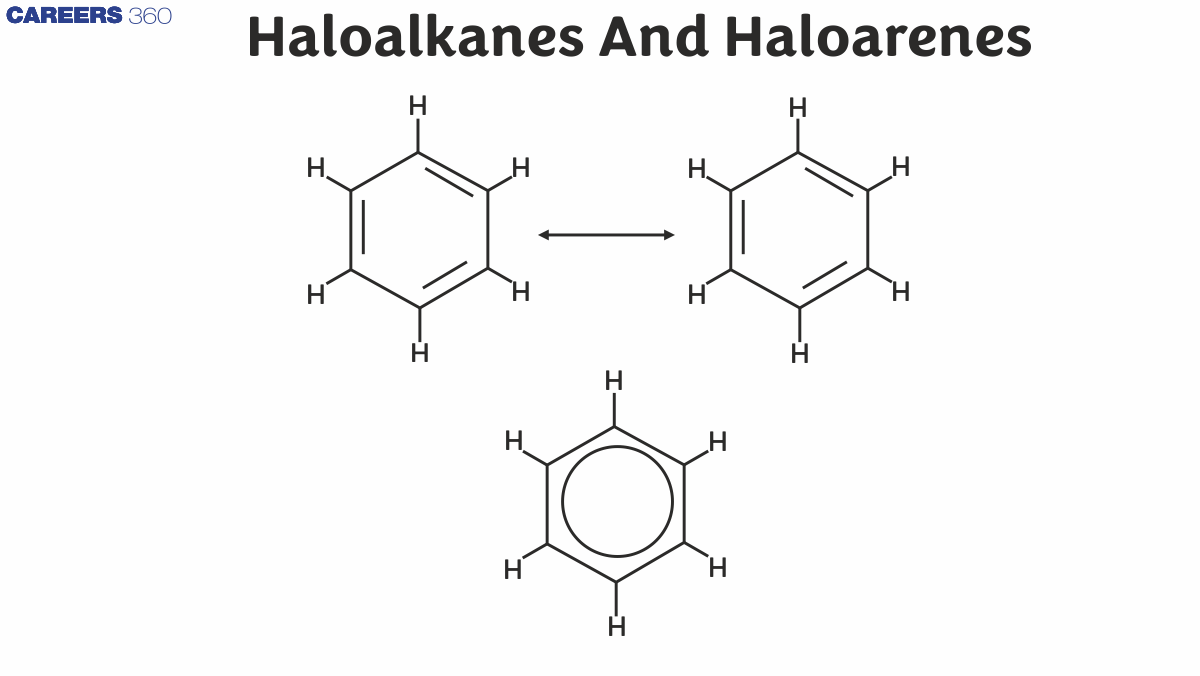
Haloarenes are aromatic compounds where one or more hydrogen atoms in an aromatic ring are replaced by halogen atoms. They are less reactive than haloalkanes due to resonance in the benzene ring, which delocalizes the electrons and stabilizes the C-X bond. Haloarenes find applications in pesticides, dyes, and pharmaceuticals.
Markovnikov Rule
Markovnikov's Rule predicts the outcome of electrophilic addition reactions to alkenes. It states that when a protic acid (HX) adds to an asymmetric alkene, the hydrogen atom attaches to the carbon with more hydrogen atoms, and the halide (or other substituent) attaches to the carbon with fewer hydrogen atoms. This rule is explained by the stability of the carbocation intermediate, which favors the more substituted and stable carbocation.
| Also read, |
Overview of the Chapter
Haloalkanes and haloarenes can be classified mainly on the basis of two aspects:
- On the basis of the number of halogen atoms
Halogens may be classified as mono, di or poly halogen compounds depending on the number of halogen compounds attached to the carbon compound.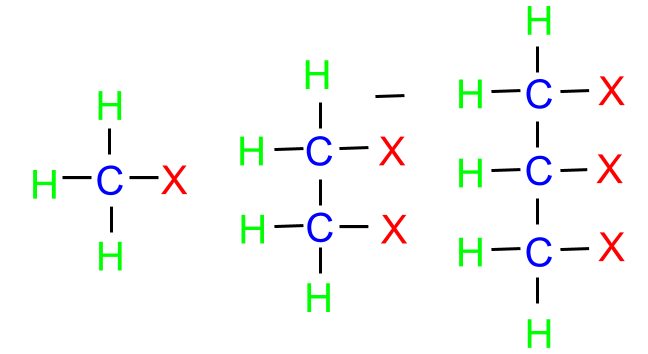
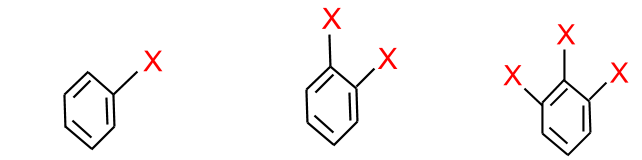
- Halogen attached to sp3 or sp2 carbon
(a) Alkyl Halide: In this class, the halogen atom is bonded to the sp3 hybridized carbon. These can further be classified into three categories as follows: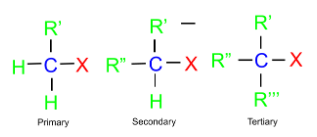
(b) Allylic Halides: In this class, the halogen atom is attached to the sp3 hybridised carbon which is attached to the carbon-carbon double bond.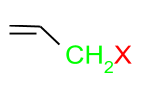
(c) Benzylic halides: These are the halides in which the halogen atom is attached to sp3 hybridised carbon which is attached to an aromatic ring.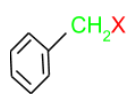
(d) Vinylic Halides: In this type of halide, the halogen atom is attached to sp2 carbon.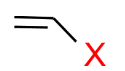
(e) Aryl Halides: In this type of halide, the halogen atom is sp2 carbon atom of an aromatic ring.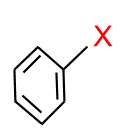
Physical properties
- Melting and boiling point: The melting and boiling point of haloalkanes and haloarenes are determined by the intermolecular forces of attraction between them. The boiling point of alkyl halides follow this order:
R-I > R-Br > R-Cl > R-F
The boiling point is always determined by vander Waal forces. As the molecular size increases, the boiling point increases. For example, in the case given below: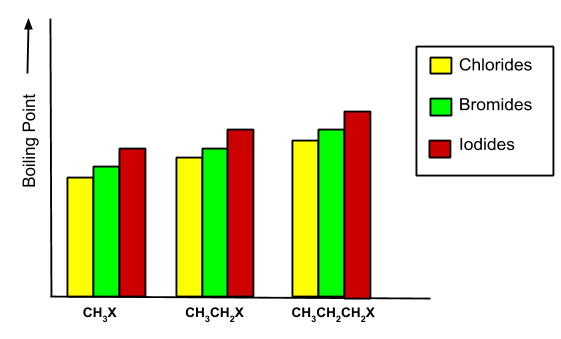
As branching increases in alkyl halides, then the boiling point decreases. - Density: The density increases as the size of the haloalkanes and haloarenes increases. It also increases with the increase of the atomic mass of halogen.
Preparation of Alkyl halides
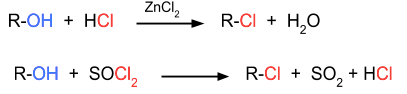
- Alkyl halides are prepared in many ways. The most common method to prepare alkyl halides is from alcohols. In this method, the alcohols are reacted with halogen acids, thionyl chloride or phosphorus halides, then the hydroxyl group is replaced with a halogen group.
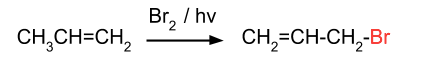
- From alkene: Alkyl halide can be prepared from alkenes by reaction with hydrogen halides or Br2/hv.
There are other important methods are also available for the preparation of alkyl halides such as, by free radical halogenation and Sandmeyer's reaction.
Chemical Reactions
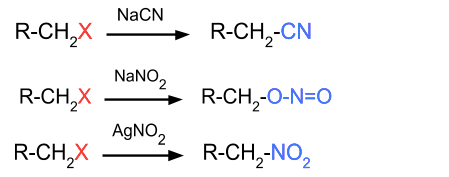
- Reaction with NaCN, AgCN, NaNO2, AgNO2: In this reaction, the halide group is replaced by CN or NO2. If alkyl halide is reacting with NaNO2 then ONO replaces the halide group but if AgNO2 is reacting then NO2 replaces the halide group.
- Finkelstein Reaction: This reaction is used for the preparation of alkyl halide from another alkyl halide. In this reaction, alkyl halide is treated with metal halide salt in the presence of acetone.
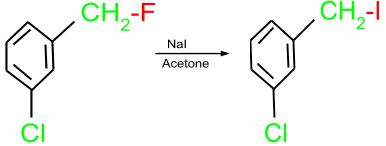
- Substitution Reactions:
Alkyl halides undergo substitution reactions either via SN1 mechanism or SN2 mechanism
(a) SN1 reaction: This mechanism is favourable when the tertiary alkyl halide is present. This mechanism occurs in two steps viz, breaking of halide bond and then the formation of the carbocation.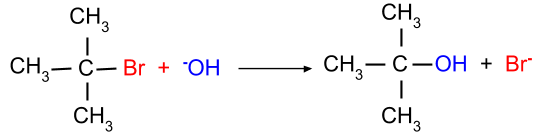
(b) SN2 reaction: This reaction is favorable when the primary alkyl halide is present. In this mechanism, the breaking of halide bond and formation of carbon-OH bond both occur at the same time. Since this reaction mechanism is bimolecular thus it is known as the SN2 mechanism.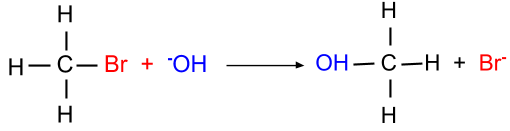
- Elimination reaction:
This reaction occurs when the alkyl halide having β-hydrogen atom is reacting with alcoholic potassium hydroxide. An example of this reaction mechanism is given below. When there is a possibility of formation of more than one alkene, then the most stable alkene is the major product.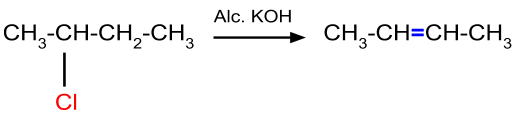
- Friedel-Crafts reactions:
These are those reactions in which an aromatic halide is treated with alkyl halide or acyl halide to attach substituents. These reactions are of two types i.e, alkylation and acylation. Both these reactions follow the electrophilic aromatic substitution mechanism. In both these reactions, the most stable product is the major product.
(a) Friedel-Crafts alkylation: In this reaction, an aromatic halide reacts with an alkyl halide and form the product as given below in reaction.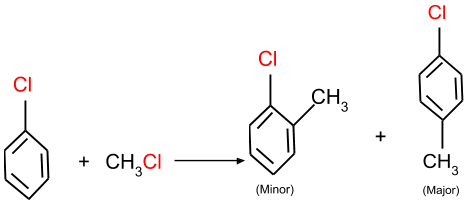
(b) Friedel-Crafts acylation: In this reaction, the aromatic halide reacts with an acyl group and form the product as given below.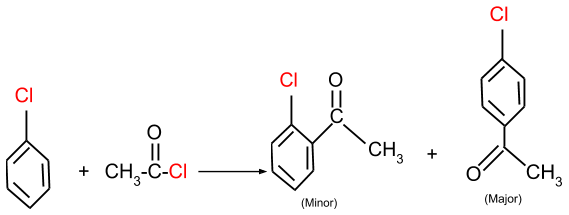
Applications of haloalkanes and haloarenes
There are some important applications of haloalkanes and haloarenes viz:
- Some haloalkanes are used as synthetic intermediate compounds in labs.
- They are used in fire extinguishers.

- The derivatives of these compounds are used for medicinal purposes.
- Some naturally occurring haloalkanes act as protective agents against ocean attackers.
- They are used as propellants.
How to Prepare for Hydrocarbons
- This chapter is part of the organic chemistry and possesses a very high weightage of marks in boards exams and other competitive exams like JEE and NEET. It is completely theory-based. You are not supposed to memorize any formula but the reactions mechanisms and named reactions are very important.
- For preparing this chapter, firstly, you must have a basic understanding of chapter - "Organic Chemistry- Some Basic Principles and Techniques". After this, you must read this chapter, "Haloalkanes and Haloarenes" from NCERT thoroughly with every concept grasp completely on your own.
- Practice lots of examples related to this chapter because then you will be able to understand the most basic concepts related to whole organic chemistry.
Prescribed Books
For this chapter, first, the NCERT book is best for initial level preparation as well as for board exams. Now, after this, if you want to prepare for competitive exams like JEE and NEET, then these are the best books for you - Morrison and Boyd and R.K Gupta by Arihant publication. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you to provide with the variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Also read,
Frequently Asked Questions (FAQs)
Haloalkanes are organic compounds in which one or more hydrogen atoms in an alkane are replaced by halogen atoms (F, Cl, Br, I). Haloarenes are aromatic compounds where halogen atoms replace hydrogen atoms in an aromatic ring.
Haloalkanes are classified based on the type of carbon atom bonded to the halogen:
- Primary: The halogen is attached to a carbon bonded to only one other carbon.
- Secondary: The halogen is attached to a carbon bonded to two other carbons.
- Tertiary: The halogen is attached to a carbon bonded to three other carbons.
Haloalkanes have higher boiling points than alkanes of similar molecular weight due to the polar nature of the C-X bond and greater molecular mass. Solubility in water is low due to their non-polar characteristics, but they are soluble in organic solvents.
Haloalkanes are used in refrigeration, solvents, anesthetics, and as intermediates in organic synthesis. Haloarenes find applications in the production of pesticides (e.g., DDT), dyes, and pharmaceuticals.
Also Read
04 Jun'25 11:17 PM
20 Dec'24 05:29 PM
13 Dec'24 09:35 AM
13 Nov'24 05:00 PM
18 Oct'24 11:58 AM
30 Sep'24 08:52 AM
17 Jun'22 05:48 PM
17 Jun'22 04:12 PM
17 Jun'22 04:06 PM