Henrys Law - Formula, Examples, Factors and FAQs
State Henry’s law
Henry’s Law can be stated as “the amount of gas absorbed or dissolved in a liquid at constant temperature is directly proportional to the pressure exerted by the gas”.
Henry’s law formula is given below.
P ∝ C
P = kh.C
In the above mathematical equation, kh in the above equation stands for Henry’s law constant being the proportionality constant for this mathematical formula or equation where,
P = Partial Pressure exerted by the
gas on liquid (atm)
kh = Henry’s law constant.
C = Dissolved gas concentration
(mol/dm-3)
Hence, the unit of kh becomes mol dm-3atm-1
The partial pressure of gas in the vapour phase is directly proportional to the mole fraction of that gas in the vapour phase.
P being the partial pressure of the gas an ‘X’ being the mole fraction, The Henry’s Law mathematical expression can also be stated as follows-
P = kh.X
William Henry - an English chemist and physician, formulated this equation in the early 19th century.
Henry’s Law Proportionality Constant is the expression used when the constant is illustrated in terms of solubility/pressure.
It is represented as ‘H’.
Henry’s Law Volatility Constant is the expression used when the constant is defined in the terms of pressure/solubility.
It is denoted by ‘kh’.
Let us take a little bit of time and think about how the pressure of the gas above a liquid can affect its solubility in that liquid.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Let us try to understand this law by considering the below example:-
Taking into consideration a very dilute solution, solvent molecules will act as near neighbours for solute molecules. the escaping probability of a particular solute particle or a molecule. Into the gaseous phase is predicted to be independent of the solute molecule concentration. The escaping rate of solute molecules will be directly proportional to the solute molecule concentration in the solution. Until the return rate becomes equal, the accumulation of solute molecules in the gas continues. The gas is very dilute in concentration, the return rate will be directly proportional to the partial pressure of the solute.
Thus, for a very dilute solution, at equilibrium with gas at low partial pressure, this partial pressure will be directly proportional to the quantity of gas absorbed or dissolved. This is Inferred as Henry’s law. However, this law is only applicable for dilute solutions and gases having low partial pressures where the amount of molecular species in the solution as well as in the gas is the same. Here it must be noted that the solubility of a gas in a liquid is greater provided that the partial pressure of the gas on liquid is greater.
Examples of Henry’s Law:-
Carbonated drinks –
Henry’s law holds in the case of carbonated drinks like Pepsi and coca-cola. The gas in the unopened carbonated drink above the liquid is generally carbon dioxide in pure form. This gas is kept at pressure little greater than atmospheric pressure in the bottle. So, the solubility of the carbon dioxide gas is high in an unopened drink. The pressurized carbon dioxide (![]() ) gas escapes into the atmosphere as soon as the bottle is reopened with a hissing sound. The decreasing partial pressure of
) gas escapes into the atmosphere as soon as the bottle is reopened with a hissing sound. The decreasing partial pressure of ![]() gas in the atmosphere leads to a decrease in the solubility of the gas present in the drink.
gas in the atmosphere leads to a decrease in the solubility of the gas present in the drink.
This is because of Henry’s law. Hence, the ![]() gas dissolved arranges itself at the surface of the drink in the form of bubbles and escape into the atmosphere. The carbonated drink when kept open for a long duration reaches equilibrium with the carbon dioxide (
gas dissolved arranges itself at the surface of the drink in the form of bubbles and escape into the atmosphere. The carbonated drink when kept open for a long duration reaches equilibrium with the carbon dioxide (![]() ) concentration in the atmosphere (0.05%) causing it to go flat (losing frizzy taste).
) concentration in the atmosphere (0.05%) causing it to go flat (losing frizzy taste).
Related Topics Link, |
Oxygenation of Blood and Respiration:-
Respiration is the process where the exchange of oxygen (![]() )
)
For carbon dioxide (![]() ) takes place for gaining energy from the breakdown of sugars.
) takes place for gaining energy from the breakdown of sugars.
This process of respiration involves the intake of oxygen called inhalation. Inhalation is a process where it is accompanied by an increase in oxygen’s partial pressure in the alveoli. When the oxygenated and deoxygenated blood comes in contact with alveoli,
Gas exchange takes place following henry’s law.
Oxygen flows from alveoli into the deoxygenated blood as the amount of dissolved oxygen in the deoxygenated blood is low and the partial pressure of oxygen in the alveoli is high.
The concentration of carbon dioxide in the deoxygenated blood is very high and the partial pressure of carbon dioxide in the alveoli is low. This causes the flow of carbon dioxide from the blood to the alveoli.
Hence, Henry’s Law holds an important position in the respiration of many organisms.
Factors Affecting Henry’s Law:-
Henry’s Law Constant is dependent upon the following factors.
Nature of gas
Nature of the solvent
Pressure and temperature
Different gases have different values of Henry’s Law Constant in different kinds of solvents.
This can be explained by Henry’s Law Graph:-
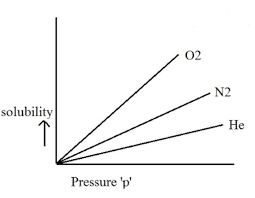
Also Read:
Limitations of Henry’s law:-
The law holds only when the molecules present in the system are in an equillibrium state.
Henry’s Law is not applicable when the gases are under very high pressure.
This law is also not applicable when the solution and gas take part in a chemical reaction with each other.
At constant pressure, Henry’s Law constant is inversely proportional to the mole fraction of the gas.
It can be represented as follows,
P ∝ X
P = kh . X
Hence, at constant pressure, the equation becomes –
X ∝ 1/kh
The above relation states that, the mole fraction value of gas in liquid increases as Henry’s Law constant decreases.
Temperature increase results in a decrease of solubility of a gas in a liquid. Thus the relation between the solubility of a gas in liquid and temperature can be given as,
T ∝ 1/solubility ---------- (1)
We are familiar with the fact that the increase in Henry’s constant results in a decrease in solubility of a gas in liquid when pressure is kept constant. So the equation can be stated as –
kh ∝ 1/solubility ---------- (2)
From equations (1) and (2),
T ∝ kh
Henry’s Law does not apply to which gas?
The dissolution of molecules of ammonia gas into the water do not follow Henry’s law. The majority of ammonia molecules (![]() ) unite with water molecules (
) unite with water molecules (![]() ) leading to the formation of
) leading to the formation of ![]() i.e. ammonium hydroxide.
i.e. ammonium hydroxide.
![]() further dissociates into
further dissociates into ![]() and
and ![]() ions.
ions.
Thus, the ions present now in the solution are – ![]() ions,
ions, ![]() ions and
ions and ![]() .
.
This results in the formation of the following equilibrium state-
Hence, we can conclude that Henrys Law does not hold for NH3 gas as this gas i in water.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The amount of gas absorbed or dissolved in a liquid at
constant temperature is directly proportional to the
pressure exerted by the gas.
Henry’s Law is only applicable for dilute solutions where the molecular species in the solution and gas is equal.
Henry’s law cannot be applied to the gases placed under very high pressure.
Henry’s Law Constant depends on the following factors-
1 . nature of gas
2 . nature of the solvent
3 . pressure and temperature.