Homolytic and Heterolytic Fission - Definition, Difference, FAQs
The covalent bond is broken by the process of bond fission by which the electron is distributed and leads to the chemical reaction. Chemical bonds can be broken in multiple ways. One of the important forms of bond fission is Homolytic and heterolytic fission.
In this article, we cover the concept of hemolytic and heterolytic bond fission, which is part of organic chemistry. It is an important topic of class 11, Joint Entrance Examination (JEE Main), and National Eligibility Entrance Test (NEET), and other entrance exams of engineering and medicine.
Homolytic fission
Homolytic fission which is also known by the name hemolysis is defined as a type of bond fission that generally involves the dissociation of a particular molecule in which each of the original fragments of the molecule retains one electron. Homolytic fission as a result gives two free radicals this process generally occurs when neutrally charged molecule undergoes homolytic fission, the result is two free radicals out of which each of the chemical species generally contains one electron which they attains from the bond pair.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Homolytic fission
- Write the heterolytic fission definition.
- Difference between homolytic and heterolytic fission
- Some Solved Example
Homolytic fission can alternatively be referred to as homolytic cleavage or bond homolysis. These phrases are taken from the Greek word 'homo,' which roughly translates to 'equal breaking.'
The homolytic bond dissociation energy of a molecule is commonly referred to as the energy required to induce homolytic fission in the molecule. Below is a diagram depicting the homolytic fission of molecule AB, which results in the creation of two free radicals (A0 and B0).
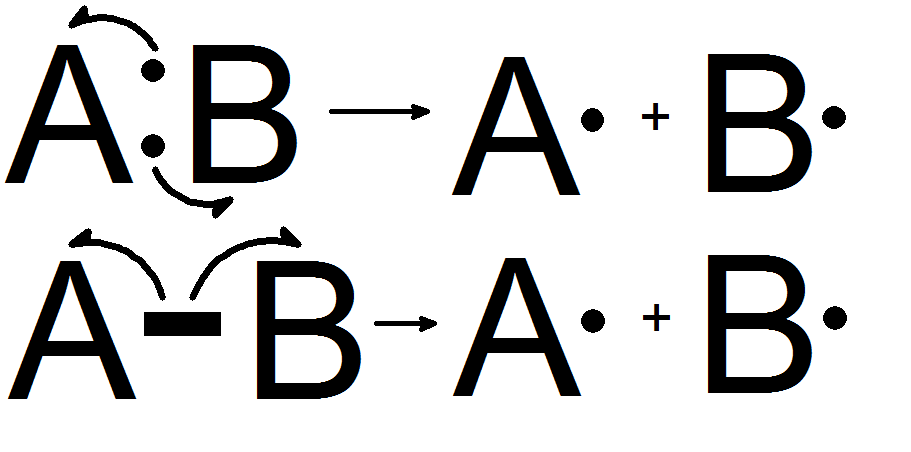
The homolytic fission of a molecule usually necessitates a significant amount of energy. This is why, as described below, this sort of bond fission occurs in only a few circumstances.
- When a molecule is exposed to UV light (the electromagnetic radiation corresponding to the ultraviolet region of the electromagnetic spectrum).
- When the requisite amount of heat is applied to the molecule in order to overcome the required bond dissociation energy for homolytic fission.
- When carbon compounds are heated to extremely high temperatures in the absence of oxygen in order to facilitate pyrolysis, the process is known as pyrolysis.
In some situations, homolytic fission can be produced by feeding the molecule with only a small amount of heat. The homolytic breakage of oxygen-oxygen bonds in peroxides is one such example. These intramolecular bonds are rather weak, meaning that their bond dissociation energies are very low. As a result, just a modest quantity of heat energy is required to overcome this barrier.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Write the heterolytic fission definition.
Heterolytic fission is also known by the name heterolysis this is defined as a type of bond fission in which a covalent bond is present between two chemical species which is broken unevenly in which one of the chemical species contains bond pair of electrons and the other left one species does not contain any of the electrons which are capable of forming the bond pair. The main products formed during heterolytic fission are neutrally charged molecules containing cations i.e. a positive charge and the other ones are those that contain negative charges known by the common name anions.
Those chemical species which are not able to retain any of the bonded electrons after the bond fission are generally known as the cation and these cations are basically said to be positively charged ions and these are the product of the heterolytic fission of any neutral molecule. On the other hand, the negatively charged heterolysis result which is also known by the name anions is defined as the chemical species which retains both bound electrons by following the bond fission process.
The word 'heterolysis' is generally taken from the Greek which has the meaning 'unequal breaking.' The other name for heterolysis is Homolytic cleavage. The following diagram represents the two methods by which any molecule AB can undergo heterolytic fission. As it is represented in the first case B is able to gain the bond pair of electrons and forms an anion while A acts as a cation. On the other in case second we can also see that A attains the bond pair and forms the anion while B acts as a cation here.
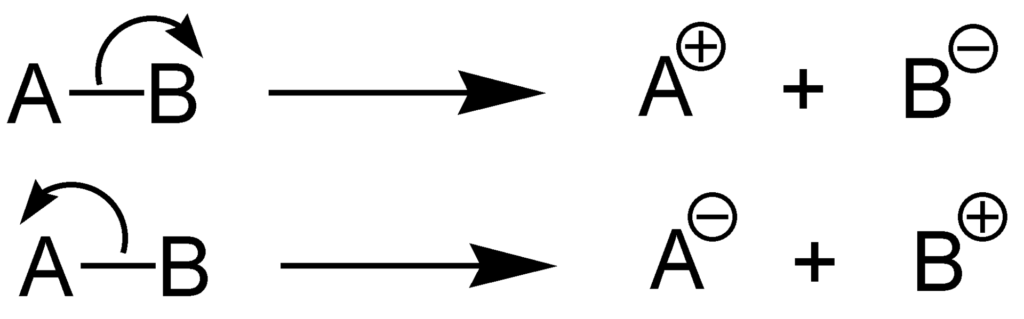
It can also be said that when any covalent bond goes through the process of heterolytic fission then those bound species that have the highest electronegativity generally keep the bond pair of electrons and attain a negative charge. On the other hand, if we consider the more electropositive species then normally these types of species do not retain any electrons, therefore they generally acquire a positive charge.
The heterolytic bond dissociation energy is the amount of energy necessary to cleave a covalent bond by heterolytic cleavage (not to be confused with homolytic bond dissociation energy). This figure is occasionally used to represent a covalent bond's bond energy.
Example of heterolytic fission:
One of the main examples of heterolytic fission is the fission of hydrogen chloride molecule which produces hydrogen ion as cation and chloride ion as anion and the reaction can be shown below.
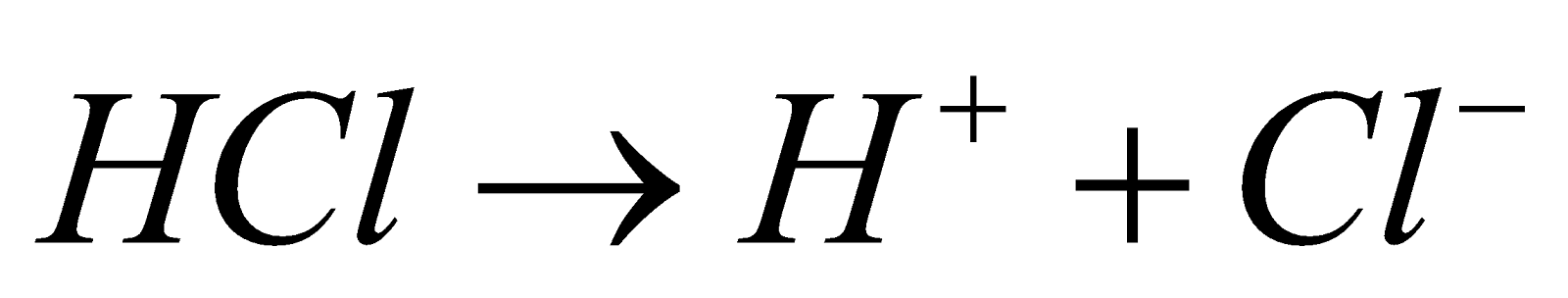
Because its electronegativity is stronger than that of hydrogen, the chlorine atom preserves the bond pair of electrons. As a result, the chloride anion and hydrogen cation are generated as products.
| Related topics, |
Homolytic and Heterolytic fission on the basis of covalent bond
When comparing the bond dissociation energies for identical types of bonds, it can be shown that the heterolytic dissociation energy is significantly larger than the homolytic dissociation energy. When a neutral molecule is hydrolyzed, two ions are produced: a positive and a negative ion. Separation of these opposing charges, on the other hand, necessitates a significant amount of energy. Bond dissociation takes a simpler path in the gas phase, namely homolysis. However, we can say that heterolysis is said to be the type of breakage in an ionizing solvent.
The destruction of a covalent chemical bond is referred to as fission. To put it another way, it splits a molecule into two moieties. Homolytic fission, which produces two equal moieties, and heterolytic fission, which produces two unequal moieties, are the two types of fission.
Also, students can refer to,
- NCERT Solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques - NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
Difference between homolytic and heterolytic fission
The breaking of a chemical link and the formation of two equal fragments is known as homolytic fission. Each fragment receives one bond electron. The “homolytic bond dissociation energy” is the amount of energy absorbed or released during homolytic fission. The breaking of a chemical connection and the formation of two unequal fragments is known as heterolytic fission. It provides two bond electrons to one fragment and none to the other. The main distinction between homolytic and heterolytic fission is this. The "heterolytic bond dissociation energy" is the amount of energy absorbed or released during heterolytic fission.
Some Solved Example
Example.1
When a covalent bond undergoes heterolytic fission, it can produce
1)cations
2)Anions
3)Free radicals
4) (correct)Both a and b
Solution
Heterolytic Covalent bond fission -
The shared pair is taken away by one of the more electronegative atoms.
Heterolytic products are cation and anion. 
Free radical is obtained when a covalent bond cleans in a homolytic manner cations and anions are formed by heterolytic fission of covalent bonds.
Therefore, option (4) is correct.
Example. 2
Free radicals can be generated by which type of covalent bond fission?
1)Heterolytic
2) (correct)Homolytic
3)both a and b
4)None of these
Homolytic Covalent bond fission -
Bonded atom takes away one e - out of the shared pair.

Upon homolytic fission of covalent bond free radicals are produced.
Therefore, option (2) is correct.
Example. 3
Ionic reactions with organic compounds proceed through :
(A) homolytic bond fission
(B) heterolytic bond fission
(C) free radical formation
(D) primary free radical
(E) secondary free radical
Choose the correct answer from the options given below :
1)(A) only
2)(C) only
3) (correct)(B) only
4)(D) and (E) only
Solution
Ionic reaction with organic compound proceeds through heterolytic bond fission.
In ionic reactions with organic compounds, bond cleavage usually occurs heterolytically, where one atom retains both electrons of the bond, forming ions. Exceptions may involve homolytic bond fission, where each atom retains one electron, common in radical reactions.
Hence, the answer is the option (3).
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Most of the chemical reactions occur by breaking or building chemical bonds. Bond fission refers to the breaking of a chemical bond (typically a covalent bond). The one of the important forms of bond fission are Homolytic and heterolytic fission.
These are the neutral intermediates, which are generated when a single bond is homolytically cleaved. In organic chemistry, a few frequent bonds that cleave to form free radicals are C-Cl, C-O, C-I, C-Br, C-H, and C-C.
The main distinction between homolytic and heterolytic fission is that homolytic fission gives each fragment one bond electron; whereas heterolytic fission offers one fragment two bond electrons and the other fragment none.
A covalent connection is created when the electrons from each participating atoms are shared equally. Shared pair or bonding pair generally represents the pair of those electrons which involves in the bonding. Molecular bonds are also known by other name i.e. covalent bonding.
Two electrons are divided between the products equally in a homolytic cleavage while in a heterolytic cleavage, a covalent bond breaks as one fragment gets both of the shared electrons.
Heterolytic fission is the process in which a covalent bond breaks unevenly, resulting in the formation of a cation and an anion. One atom takes both of the shared electrons, while the other atom is left with none.
Example of heterolytic fission can be seen in the breaking of the hydrogen chloride (HCl) molecule. If the H-Cl bond breaks heterolytically, the hydrogen atom takes both electrons, forming a hydride ion (H−), while the chlorine atom becomes a positively charged cation (Cl+)
The differences are:
- Products: Homolytic fission produces free radicals, while heterolytic fission produces ions (cations and anions).
- Electron Distribution: In homolytic fission, each atom retains one electron from the bond, whereas in heterolytic fission, one atom retains both electrons.
- Bond Type: Homolytic fission often occurs in non-polar covalent bonds. In contrast, heterolytic fission is common in polar covalent bonds due to the differing electronegativities of the atoms involved.
The type of fission can be influenced by several factors, including temperature and the polarity of the solvent. Generally, high temperatures may promote homolytic fission because of the energy required to break the bonds. Meanwhile, polar solvents might stabilize ions, favoring heterolytic fission.
Also Read
12 Dec'24 04:49 PM
04 Nov'24 05:31 PM
21 Oct'24 05:53 PM
09 Oct'24 06:12 PM
09 Oct'24 04:00 PM
30 Sep'24 10:57 AM
30 Sep'24 08:52 AM

