Hydrogen Bonding
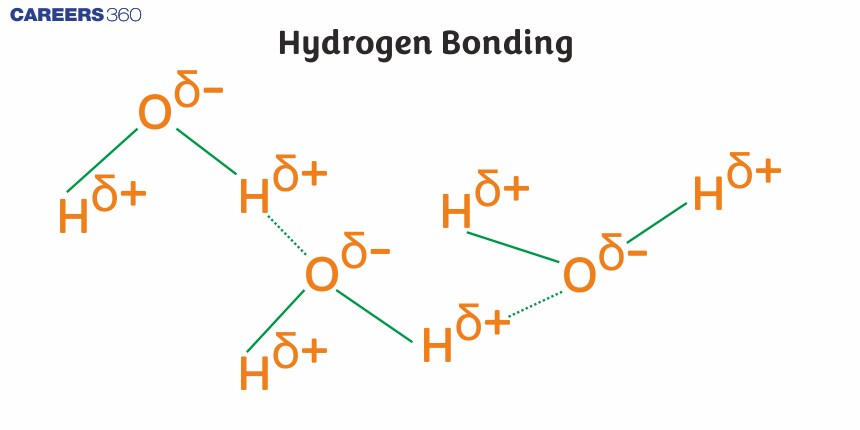
Hydrogen bonding has immense effects on the features of substances. It is a shared interaction of a hydrogen atom that is covalently bonded to an electronegative atom, for instance, oxygen, nitrogen, or fluorine, with another electronegative atom. It is not a real chemical bond at all but rather a specially strong kind of intricate dipole-dipole attraction. Because of hydrogen bonds, various structures of water, proteins, and nucleic acids like DNA also show features of stability.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Hydrogen Bonding
- Formation of Hydrogen Bond
- Types of Hydrogen Bonding
- Relevance and Applications of Hydrogen Bonding
- Some Solved Examples
- Summary
Hydrogen Bonding
Hydrogen bonds are strong forces that occur when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom such as O, N, F, etc. Greater the electronegativity of the atom will increase in hydrogen-bond strength. The hydrogen bond is a stronger intermolecular force, but it is weaker than a covalent or an ionic bond. Hydrogen bonds are responsible for holding together DNA, proteins, and other macromolecules.
Formation of Hydrogen Bond
A hydrogen bond is an electromagnetic attraction that occurs between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative atom. A hydrogen bond is a type of dipole-dipole interaction; it is not a true chemical bond. This hydrogen bond attraction can occur between different molecules (intermolecularly) or within different parts of a single molecule (intramolecularly).
Types of Hydrogen Bonding
There are two types of hydrogen bonding, i.e:
Intermolecular Hydrogen Bonding: Intermolecular hydrogen bonding occurs when the H-atom of one molecule and an electronegative atom of another molecule are close to each other. For example, hydrogen bonds between the molecules of hydrogen fluoride. Intermolecular hydrogen bonding results in the association of molecules. Thus, it increases the melting point, boiling point, solubility, etc.
Intramolecular Hydrogen Bonding: Intramolecular hydrogen bonding occurs when the hydrogen atom and an electronegative atom of the same molecule are close to each other. Intramolecular hydrogen bonding results in the cyclization of the molecules and prevents their association. Thus, the properties of these compounds like melting point, boiling point, etc. are usually low. For example, intramolecular hydrogen bonding is present in molecules such as o-nitrophenol, o-nitrobenzoic acid, etc..
Relevance and Applications of Hydrogen Bonding
Hydrogen bonding relevance has gone on to influence many real applications and academic fields.
1. Biological Importance: Of biological significance is the role of these bonds in ensuring that macromolecules remain stable throughout their functional processes. For instance, hydrogen bonds hold the two strands of DNA in a double helix configuration through the hydrogen bonding between complementary base pairs such as adenine-thymine and guanine-cytosine. They assume great importance in the processes of replication and transcription, these latter processes ensuring that the transmission of genetic material is carried out accurately. Hydrogen bonds help in the folding of proteins for structural stability and function.
2. Water Properties: All these aspects—the high heat capacity, cohesion, and adhesion of a substance like water—add up to the importance of the element for life because of quite rare liquid water as a result of the hydrogen bonding it allows. These are for the sustention, and creation of life, and to help in the facilitation of chemical reactions in the biological system.

3. Environmental science: Hydrogen bonding explains various environmental processes related to the solubility of gases in water and the behavior of certain pollutants. Hydrogen bonds affect the ability of water to be a solvent in different substances, thus impacting the dispersion and concentration of pollutants in various aquatic systems.
Of course, hydrogen bonding stands for one of the most unifying concepts beneath a broad horizon of phenomena, both natural and man-made, cutting across an enormous range of impacts from scientific disciplines like biology to materials science. This proves how important understanding molecular interactions and the behavior of substances is.
Recommended topic video on (Hydrogen bonding)
Some Solved Examples
Example 1
Question: Which of the following compounds has the least tendency to form hydrogen bonds?
1) HF
2) HCl
3) H2O
4) NH3
Solution: Hydrogen bonding occurs when a hydrogen atom is bonded to a highly electronegative atom like F, O, or N. These elements can form hydrogen bonds due to their high electronegativity. In the given options, HCl has chlorine, which is less electronegative than F, O, and N. Therefore, HCl has the least tendency to form hydrogen bonds. Hence, the answer is option (2) HCl.
Example 2
Question: HF has the highest boiling point among hydrogen halides because it has:
1) Strongest Van der Waals interaction
2) Lowest ionic character
3) Strongest hydrogen bonding
4) Lowest dissociation enthalpy
Solution: HF exhibits strong hydrogen bonding due to the high electronegativity of fluorine. This strong hydrogen bonding leads to a higher boiling point compared to other hydrogen halides like HCl, HBr, and HI, which primarily rely on weaker Van der Waals forces for intermolecular attractions. Therefore, the reason HF has the highest boiling point is due to its strongest hydrogen bonding. Hence, the answer is option (3) Strongest hydrogen bonding.
Example 3
Question: Which of the following hydrogen bonds is the strongest?
1) O-H---F
2) O-H---H
3) F-H---F
4) O-H---O
Solution: The strength of hydrogen bonds depends on the electronegativity of the atoms involved. Fluorine is the most electronegative element, making F-H---F the strongest hydrogen bond among the given options. Therefore, the strongest hydrogen bond is option (3) F-H---F.
Example 4
Question: The reason for the exceptionally high boiling point of water is:
1) Its high specific heat
2) Its high electric constant
3) Low ionization of water molecule
4) Hydrogen bonding in the molecule of water
Solution: Water has a high boiling point due to the presence of strong intermolecular hydrogen bonds. These hydrogen bonds result in a significant amount of energy being required to break them, leading to an exceptionally high boiling point. Thus, the reason for the high boiling point of water is option (4) Hydrogen bonding in the molecule of water.
Example 5
Question: Methanol and ethanol are miscible in water due to:
1) Covalent character
2) Hydrogen bonding character
3) Oxygen bonding character
4) None of these
Solution: Methanol and ethanol are miscible in water because they can form hydrogen bonds with water molecules. The hydroxyl group (-OH) in methanol and ethanol allows them to interact strongly with the water molecules, leading to complete miscibility. Therefore, the reason for their miscibility in water is option (2) Hydrogen bonding character.
Summary
One of the most important types of intermolecular forces is hydrogen bonding, characterized by an interaction of a hydrogen atom covalently attached to a very electronegative atom with another electronegative atom. Among characteristic properties, strength is envisaged to fall between the strongly bonded covalent and the much weaker Van der Waals forces. Further subdivision can be done into an intermolecular and intramolecular variety of hydrogen bonds; these participate massively in many different chemical and biological processes.
Also Read
19 Feb'25 11:09 AM
07 Feb'25 10:38 AM
07 Feb'25 10:22 AM
07 Feb'25 10:18 AM
07 Feb'25 10:16 AM
07 Feb'25 10:11 AM
18 Oct'24 05:56 PM
18 Oct'24 10:52 AM
10 Oct'24 12:05 AM
09 Oct'24 11:41 PM