Hydrogen - Notes, Topics, Books, FAQs
Hydrogen is the smallest and lightest element in the universe among all the elements discovered so far. It has one electron and one proton. It has three isotopes: protium, deuterium, and tritium. Hydrogen is also an important part of many biomolecules, such as proteins, DNA, carbohydrates, etc. In nature, it is available as natural gas, but in pure form, it is highly inflammable. In the modern periodic table, its position is not fixed because it belongs to the s-block, but its properties resemble those of non-metals.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Hydrogen- Important Topics
- Overview Of The Chapter
- Hydrides
- Physical Properties
- Chemical Properties
- Dihydrogen As a Fuel
- How To Prepare For Hydrogen?
- Prescribed Books
Hydrogen- Important Topics
Isotopes of Hydrogen:
Hydrogen has three isotopes: protium, 1H, deuterium, 2H or D, and finally, tritium, 3H or T. The atoms of these isotopes differ only in their neutron count and share identical electron configurations. And tritium is the only radioactive isotope of hydrogen
Dihydrogen:
Dihydrogen is a colorless, odorless, tasteless, combustible gas. It is lighter than air and insoluble in water. The electronegativity of hydrogen is in between metals and non-metals so it behaves as both electropositive and electronegative.
Hydrides:
Hydrides are those compounds that contain hydrogen bonded to some other more electropositive element. They are colorless or greyish crystalline solids and have high melting and boiling points.
Importance Of Water (H2O):
Water is known for having solvent capabilities, and colloquially it is often called the "universal solvent" because it dissolves more substances than any other liquid. Water is neutral in nature. pH of the pure water is 7. It is a weak electrolyte and ionizes into H+ and OH- ions.
Hard Water And Soft Water:
When water has a high concentration of dissolved minerals—mostly calcium and magnesium and when it produces sufficient lather with soap that is called Hard Water And Soft Water respectively.
Hydrogen Peroxide:
Hydrogen Peroxide has a formula H2O2 it is a colorless, unstable, and highly reactive liquid at room temperature. It is also an important chemical used in pollution control treatment of domestic and industrial effluents.
Hydrogen has various important real-life applications as mentioned below:
- In petroleum refineries, it is used to remove sulfur content. In these plants, it is also used for hydroisomerization.

- In ultraviolet lamps, it is used in the form of deuterium. These lamps on heating produce light in the ultraviolet region.

- One of the first uses of hydrogen is its use in gas balloons. Due to its light weight, it is used for flying hot balloons.

Overview Of The Chapter
Hydrogen is one of the important element in the universe. In nature, it exists in dihydrogen form as H2. In this article, you will study the various insights about this chapter and preparation tips.
The Position Of Hydrogen In The Periodic Table:
Hydrogen is placed in the first position of the periodic table. However, its actual position is always has been a matter of discussion in science. Its electronic configuration is 1s1, which means either it requires one more electron to completely fulfill the s orbital or it can lose one electron. Thus Hydrogen behaves like alkali metals and halogens. Like alkali metals, Hydrogen forms halides, oxides, and sulfides. But in terms of ionization enthalpy, Hydrogen resembles more to halogens than alkali metals.
Isotopes Of Hydrogen:
As you all must know from your earlier classes, Hydrogen has three isotopes: protium, deuterium and tritium. Protium has no neutron, deuterium has 1 neutron and tritium has 2 neutrons. Since the electronic configuration of all these isotopes is same therefore chemical properties of these isotopes are same. However, their physical properties are considerably different from each other.
Dihydrogen
Dihydrogen is one of the most important chemicals in the universe. It is the most abundant element in the universe with almost 70% mass of the universe. In this section, you will study the preparation of dihydrogen, its properties, and uses.
- Preparation:
Hydrogen is prepared by the reaction of granulated zinc with hydrochloric acid or with aqueous alkali.
$\\*Zn\: +\: 2H^{+}\: \rightarrow \: Zn^{2+}\: H_{2}\\*Zn\: +\: 2NaOH\: \rightarrow \: Na_{2}ZnO_{2}\: +\: H_{2}$ - Properties of Dihydrogen
(i) Physical properties:
(a) It is colourless, tasteless, combustible gas.
(b) It is almost 1/4 times lighter than air.
(c) It is very low solubility in water.
(ii) Chemical Properties:
The bond dissociation enthalpy of dihydrogen is very high thus, it is very inert at room temperature. - Reaction with halogens:
It reacts with halogens and forms hydrogen halides of the form HX.
$H_{2}(g)\: +\: X_{2}(g)\: \rightarrow\: 2HX(g)$ - Reaction with dioxygen:
It reacts with dioxygen and forms water.
$2H_{2}(g)\: +\: O_{2}(g)\: \rightarrow\: 2H_{2}O(l)$ - Reaction with dinitrogen:
It reacts with dinitrogen and forms ammonia:
$3H_{2}(g)\: +\: N_{2}(g)\: \rightarrow\: 2NH_{3}(g)$ - Reaction with metals:
It reacts with metals and forms hydrides
$H_{2}(g)\: +\: 2M(g)\: \rightarrow\: 2MH(s)$ - Uses
(i) It is used in the synthesis of ammonia.
(ii) It is used in the manufacture of vanaspati fat.
(iii) It is used in the production of metal hydrides.
(iv) It is also used for the synthesis of hydrogen chloride.
Hydrides
Water
Water is present on the earth in a tremendous amount. In the human body, it is available up to 70% and in some fruits like watermelon, it is present up to 96%. On earth, it is present in various forms as given in the table:
Source | Total(%) |
Oceans | 97.33 |
Saline lakes and inland seas | 0.008 |
Polar ice and glaciers | 2.04 |
Groundwater | 0.61 |
Lakes | 0.009 |
Soil moisture | 0.005 |
Atmospheric water vapour | 0.001 |
Rivers | 0.0001 |
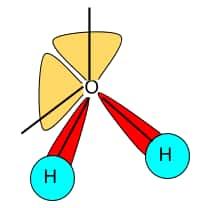
Water has the bent structure with 104.50 bond angle and 2 lone pair of electrons on the oxygen atom. It is a highly polar molecule and forms hydrogen bonding with other water molecules. The structures of water are depicted in fig below: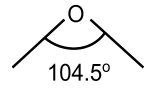
Physical Properties
- It is a colourless liquid.
- It's melting point is 0oC and boiling point is 100oC.
- It is an excellent solvent for the transportation of ions and molecules.
- It has a high heat of vaporization and high heat capacity .
- It has higher specific heat, thermal conductivity, surface tension, etc.
Chemical Properties
- Amphoteric nature
Water is amphoteric in nature. Thus it has the ability to act as an acid as well as a base. - Redox reactions with water
Water is reduced very easily on reacting with highly electropositive metals.
$2H_{2}O(l)\: +\: 2Na(s)\: \rightarrow \: 2NaOH(aq)\: +\: H_{2}(g)$ - Hydrolysis reaction
Some covalent and ionic compounds are hydrolysed in water.
$\mathrm{P_{4}O_{10}(s)\, +\, 6H_{2}O(l)\, \rightarrow \, 4H_{3}PO_{4}(aq)}$
Hard and Soft Water
When salts of calcium and magnesium are dissolved in the water then it is called as hard water, but when these salts are not present in the water then it is known as soft water. Hard water does not form lather with soaps but soft water forms lather. For washing in hard water, soaps are not efficient and thus we use detergents. With proper water treatment, this hardness of water can be removed.
Hydrogen Peroxide(H2O2)
Hydrogen peroxide is one of the most important compounds which is used in pollution control treatment. Some of the important physical properties of hydrogen peroxide are given in the table below:
Melting Point(K) | 272.4 |
Boiling Point(K) | 423 |
Vapor pressure | 1.9 |
Density | 1.64 |
Dielectric Constant | 70.7 |
Electrical Conductivity | 5.1x10-8 |
- Preparation: Hydrogen peroxide is prepared by the following methods as discussed below:
(i) In this method, barium peroxide is treated with sulphuric acid and thus form barium sulphate and hydrogen peroxide.
$\mathrm{BaO_{2}.8H_{2}O(s)\: +\: H_{2}SO_{4}(aq)\: \rightarrow\: BaSO_{4}(s)\: +\: H_{2}O_{2}(aq)\: +\: 8H_{2}O(l)}$
(ii) In this method, with the help of electrolytic oxidation of acidified sulfate solutions, peroxodisulphate is obtained. This peroxodisulphate is then hydrolyzed to yield hydrogen peroxide.
$\mathrm{2HSO_{4}^{-}(aq)\, \overset{Electrolysis}{\rightarrow}\, HO_{3}SOOSO_{3}H(aq)\overset{Hydrolysis}{\rightarrow}\, 2HSO_{4}^{-}(aq)\, +\, 2H^{+}(aq)\, +\, H_{2}O_{2}}$ - Uses
(i) In daily life, hydrogen peroxide is used as a hair bleach.
(ii) It is used in the synthesis of tartaric acid and some food products.
(iii) It is used a bleaching agent for textiles, leather, fats,etc.
(iv) It is used to manufacture chemicals like sodium perborate and per-carbonate.
Dihydrogen As a Fuel
Dihydrogen can be used fuel as on combustion it produces a tremendous amount of heat. The combustion of dihydrogen can produce almost three the energy as produced by petrol. Also, the pollutants released are also lesser than petrol. The only pollutant from dihydrogen released is oxides of dinitrogen, but this problem can be solved very easily by using a little amount of water. This addition of water will reduce the temperature and thus the reaction between nitrogen and oxygen will not take place.
But using dihydrogen gas as a fuel has some limitations. The cylinder of compressed dihydrogen gas weighs almost 30 times heavier than a petrol tank. Also, converting dihydrogen to the liquid state requires cooling to 20K. Thus it requires expensive insulated tanks.
How To Prepare For Hydrogen?
This chapter is a part of inorganic chemistry. It is completely theory-based and very easy to learn, there is no need to memorize any formula.
Before reading this chapter, first, you must have the basic knowledge of the chapter - Periodic classification of elements.
Rest this complete chapter is very simple, just be regular and be consistent in your numerical practice.
Prescribed Books
First, you must finish the class XI and XII NCERT textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow O.P. Tandon. You must definitely solve the previous year's papers. Meanwhile, in the preparation, you must continuously give the mock tests for the depth of knowledge. Our platform will help you with a variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Also Read,
Frequently Asked Questions (FAQs)
Hydrogen can be produced through various methods, including:
- Steam Methane Reforming (SMR): A process that extracts hydrogen from natural gas.
- Electrolysis: Splitting water into hydrogen and oxygen using electricity.
- Gasification: Converting organic or fossil-based materials into hydrogen and other products.
- Biomass Gasification: Producing hydrogen from organic materials through thermal or chemical means.
Hydrogen has numerous applications, such as:
- Fuel Cells: Providing clean energy for vehicles and stationary power generation.
- Industrial Processes: Serving as a feedstock in the production of ammonia, petroleum refining, and food processing.
- Energy Storage: Storing excess renewable energy and supplying it when needed.
- Metal Processing: Used in various metallurgical processes to remove impurities.
Hydrogen can be considered a clean energy source when produced through renewable methods, such as electrolysis powered by solar or wind energy. Fuel cells emitting only water vapor as a byproduct also contribute to reducing greenhouse gas emissions. However, the environmental impact depends on the production method used.
Yes, hydrogen can power vehicles equipped with fuel cells, which convert hydrogen and oxygen into electricity to power electric motors. Hydrogen fuel cell vehicles (FCVs) offer a zero-emission alternative to traditional gasoline or diesel vehicles.
Also Read
19 Feb'25 10:58 AM
07 Feb'25 10:09 AM
19 Dec'24 09:54 PM
07 Oct'24 02:52 PM
07 Oct'24 02:41 PM
04 Oct'24 07:50 PM
04 Oct'24 07:31 PM
Articles
Questions related to
Correct Answer: 1:4
Solution : The correct option is 1:4.
If the density of oxygen is 16 times that of hydrogen, the ratio of their velocities of sound will be the same as the ratio of their densities
The velocity of sound from oxygen to hydrogen is 1:4.
v=√γP/ρ.
- v is the speed of sound in the gas,
- γ is the heat capacity ratio
- P is the pressure of the gas, and
- ρ is the density of the gas.
Vo2/VH2 = ρH2/ρO2
√1/16 = 1/4.
Correct Answer: between 4 and 7
Solution : The correct choice is the fourth option.
Explanation: According to the passage, the pH value of human skin is generally in a range between 4.0 and 7.0, depending on various factors like body parts, age, genetics, ethnicity, and environmental conditions.
Therefore, the correct answer is, between 4 and 7.
Correct Answer: Neither I nor II follows
Solution : According to the above-given statement –
Conclusion I: North Korea will declare a state of war against the US and its ally countries soon – Though North Korea has successfully tested a hydrogen bomb, it does not imply that North Korea will declare a state of war as it could be for any other purpose as well.
Conclusion II: The US and its ally countries should not worry about North Korea's successful test of a Hydrogen bomb as it has only increased its nuclear arsenal – It's not confirmed from the above statement that North Korea will never attack the US and its ally countries and this test has only been done to increase its nuclear arsenal. Any motive can be there behind this successful test.
Therefore, the correct answer is neither conclusion I nor conclusion II. Hence, the third option is correct.
Correct Answer: Only II
Solution : The correct option is Only II.
Carbon dioxide (CO2) is classified as an air pollutant when it is found in high concentrations in the Earth's atmosphere, causing harmful impacts on the ecosystem, and climate. Human actions such as fossil fuels, have considerably boosted its concentration. The fundamental issue with increasing CO2 levels as an air pollutant is its role as a greenhouse gas, which leads to global warming.
Correct Answer: 4
Solution : The correct answer is 4.
Comprising one carbon atom and four hydrogen atoms, methane (CH4) is a colourless, odourless and extremely combustible gas. Burning it with oxygen yields carbon dioxide and water vapour. It can be made synthetically or naturally.