Hyperconjugation - Effects, Definition, Examples, Applications, FAQs
Hyperconjugation is an important concept in organic chemistry that describes the delocalization of electrons in a molecule. This delocalization occurs through the interaction between sigma bonds (usually C−H or C−C) and an adjacent empty or partially filled p-orbital, π-orbital, or a positively charged center. Hyperconjugation helps to stabilize the molecule by dispersing charge over multiple atoms.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Hyperconjugation
- Application of Hyperconjugation
- Types of hyperconjugation
- Some Solved problems
In this article, we will cover the topic (Hyperconjugation). This topic falls under the broader category of (Some basic concepts of organic chemistry), which is a crucial chapter in (Class 11 Chemistry). It is not only essential for board exams but also for competitive exams like the JEE Mains Exam ), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more
Definition and Mechanism
- Hyperconjugation: Also known as no-bond resonance, hyperconjugation is the stabilizing interaction that results from the overlap of sigma (σ) bonds with an adjacent empty or partially filled p-orbital or π-orbital.
- Mechanism: Involves the interaction of electrons in a sigma bond (usually C−H or C−C) with an adjacent empty or partially filled p-orbital or π-orbital, leading to the delocalization of electrons.
Hyperconjugation
Hyperconjugation is a general stabilizing interaction. It involves the delocalization of σ electrons of the C−H bond of an alkyl group directly attached to an atom of an unsaturated system or to an atom with an unshared p orbital. The σ electrons of the C−H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. Hyperconjugation is a permanent effect.
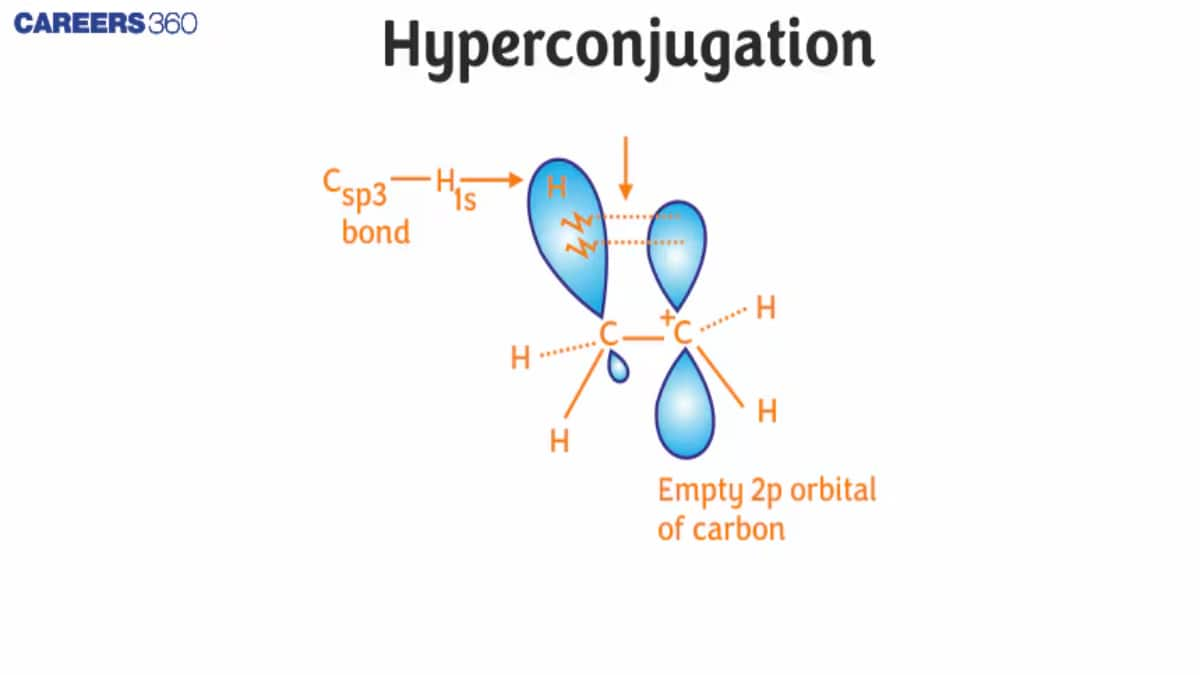
To understand the hyperconjugation effect, let us take an example of CH3CH2+ (ethyl cation) in which the positively charged carbon atom has an empty p orbital. One of the C-H bonds of the methyl group can align in the plane of this empty p orbital and the electrons constituting the C−H bond in the plane with this p orbital can then be delocalized into the empty p orbital as shown in the figure given below:

Application of Hyperconjugation
Hyperconjugation is useful in explaining the stability:
- Stability of Alkenes
- Alkyl Carbocation stability
- Alkyl Free Radicals stability
The overlapping stabilizes the carbocation in such a way that electron density from the adjacent σ bond helps in dispersing the positive charge.

In general, greater the number of alkyl groups attached to a positively charged carbon atom, the greater is the hyperconjugation interaction and stabilization of the cation. Thus, we have the following relative stability of carbocations:

Hyperconjugation is also possible in free radicals, alkenes, and alkyl arenes.
In general, the greater the number of hyperconjugative structures, the greater the stability.
Types of hyperconjugation
Positive hyperconjugation: when empty p orbital interacts with the adjacent sigma bond.
Negative hyperconjugation: when the filled p or pi orbital interacts with adjacent antibonding sigma orbital is said to negate the type of hyperconjugation.
Sacrificial Hyperconjugation
In the sacrificial Hyperconjugation, the canonical forms involve no bond resonance. The main form has no charge distribution. This types of hyperconjugation occur least but the isovalent type of hyperconjugation which is occur in free radicals and carbonation occurs readily.
Also read -
Some Solved problems
Q.1 Hyperconjugation is also known as :
(1) No bond resonance
(2) sp3−p conjugation
(3) σ−bond resonance
(4) All of these
Solution:
As we have learned
Hyperconjugation, σ-π resonance, or Baker and Nathan effect -
The conjugation between αC−Pn the sigma bond and adjacent multiple bond or carbocation is called hyperconjugation.
It is a permanent effect.
Since there is no definite bond between the carbon atom & one of the hydrogen atoms in the hyperconjugation forms, hyperconjugation is also called no-bond resonance.
Hence, the answer is the option (4).
Q.2 Which one of the following carbocations is most stable?
(1)

(2)

(3)

(4)

Solution:
As we have learned
Application of Hyperconjugation -
More the no of Hyperconjugation structure more the stability of carbocation
In (CH3)3C◻ , carbocation has 9α-hydrogen in all other options carbocation has less than 9α−H .
So (CH3)3C⊕ is most stable due to more number of hyperconjugative structures.
Hence, the correct answer is Option (3)
Q.3 Which of the following is not an application of hyperconjugation?
1)Stability of Carbocations
2)Stability of carbon-free radicals
3)Stability of Alkenes
4) (correct)Stability of Carbanions
Solution
Hyperconjugation affects the stability of Carbocations, Carbon free radicals and alkenes.
It has no effect on the stability of the carbanions.
Hence, the answer is the option (4).
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The effect in which an electron releases by an alkyl group attached to a saturated group is called hyperconjugation effect.
Hypermethylation in chemistry is the addition of excess numbers of methyl groups in an organic compound. Whereas, in biology it has a different definition, hypermethylation is a genetic disease in which epigenetic methylation of cytosine and adenine residues accumulate in the DNA.
Hyperconjugation is possible in carbocations, free radicals and alkenes. This effect was first observed by Nathan and Baker in 1935.
Hyperconjugation involves the delocalization of electrons of the carbon-hydrogen bond in an alkyl group directly attached to an unsaturated compound or to an atom with an unshared P orbital. Electrons in the carbon-hydrogen bond of the alkyl group enter into the partial conjugation with the attached unsaturated compound.
Conjugation effect is an effect in which molecular orbitals are conjugated to new molecular orbitals that are more delocalized and also lower in energy. The electrons can move freely in this new extended system.
Iso valent conjugation and sacrificial conjugation are the types of hyperconjugation in organic chemistry.
Hyperconjugation involves the interaction of sigma and π-bonds, while resonance involves the delocalization of pi electrons across adjacent atoms with p-orbitals. Hyperconjugation usually results from the interaction of single bonds, while resonance pertains to double bonds or lone pairs.
Hyperconjugation can help stabilize the double bond by allowing the π bond to interact with adjacent sigma bonds. This stabilizing effect is particularly important in determining the stability of different alkene isomers, where more substituted alkenes (with more hyperconjugative interactions) are generally more stable.
Hyperconjugation helps to explain why some alkenes are more stable than others. And also hyperconjugation has different synthetic applications such as the Anomeric effect, Gauche effect, and relative stability of carbocations.
Hyperconjugation can be identified by examining molecular structures for adjacent C-H or C-C bonds and looking for π-bonds that can stabilize nearby carbocations or radicals. Drawing resonance structures or indicating interactions between bonds can illustrate how hyperconjugation works in a given compound.
Also Read
12 Dec'24 04:49 PM
04 Nov'24 05:31 PM
21 Oct'24 05:53 PM
09 Oct'24 06:12 PM
09 Oct'24 04:00 PM
30 Sep'24 10:57 AM
30 Sep'24 08:52 AM