Inductive Effect
(−CH3,−C2H5)The inductive effect refers to the phenomenon whereby a permanent dipole arises in a given molecule due to the unequal sharing of the bonding electrons in the molecule. This effect can arise in sigma bonds, whereas the electromeric effect can only arise in pi bonds.When an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon-chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Inductive Effect
- Types of Inductive Effect
- Solved Examples Based on Inductive Effect
- Conclusion
Inductive Effect
When a covalent bond is formed between atoms of different electronegativity, the electron density is more towards the more electronegative atom of the bond. Such a shift of electron density results in a polar covalent bond. Bond polarity leads to various electronic effects in organic compounds.
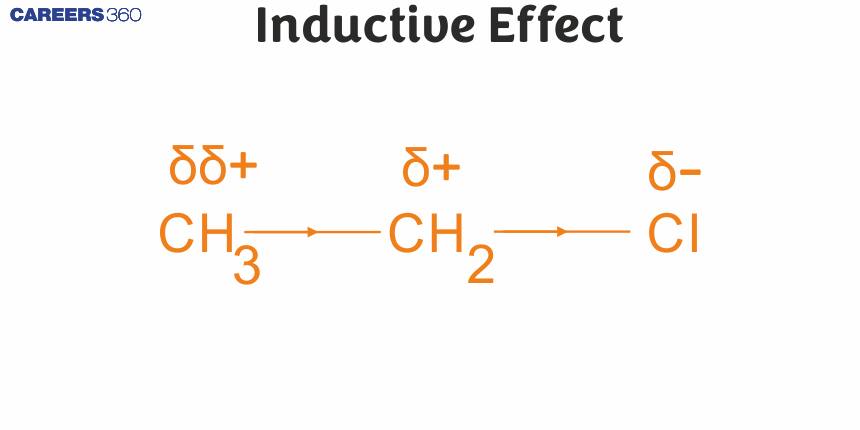
Let us consider cholorethane (CH3CH2Cl) in which the C−Cl bond is a polar covalent bond. It is polarised in such a way that the carbon-1 gains some positive charge (δ+) and the chlorine some negative charge (δ–). The fractional electronic charges on the two atoms in a polar covalent bond are denoted by symbol δ (delta) and the shift of electron density is shown by an arrow that points from δ+ to δ– end of the polar bond.
2δC2+H31C+H2Cl
In turn carbon-1, which has developed partial positive charge (δ+) draws some electron density towards it from the adjacent C-C bond. Consequently, some positive charge (δδ+) develops on carbon-2 also, where δδ+ symbolises relatively smaller positive charge as compared to that on carbon – 1.
In other words, the polar C – Cl bond induces polarity in the adjacent bonds. Such polarisation of σ- bond caused by the polarisation of adjacent σ-bond is referred to as the inductive effect. This effect is passed on to the subsequent bonds also but the effect decreases rapidly as the number of intervening bonds increases and becomes vanishingly small after three bonds.
The inductive effect is related to the ability of substituent(s) to either withdraw or donate electron density to the attached carbon atom. Based on this ability, the substitutents can be classified as electron-withdrawing or electron-donating groups relative to hydrogen.
(1) Electron Withdrawing Groups: Halogens and many other groups such as nitro(−NO2), cyano (−CN), carboxy (−COOH), ester (COOR), aryloxy (-OAr, e.g. −OC6H5),etc. are electron-withdrawing groups.
(2) Electron Donating Groups: Alkyl groups like methyl (−CH3) and ethyl (−CH2−CH3) are electron-donating groups.
The decreasing -I effect or increasing +I effect order is as follows:
Types of Inductive Effect
Inductive Withdrawal Effect (−I):- Atom or group which is more electronegative than hydrogen like halogens F1Cl,Br, I and nitro groups NO2They withdraw electrons from the adjacent carbon atom creating partial positive charge.
Electron-Donating Effects (+I): Atoms or groups, which are low-electronegativity and have electron-donating properties such as alkyl groups
−NH3+>−NO2>−SO2R>−CN>−SO3H>−CHO>−CO>−COOH>−F>−COCl>−CONH2>−Cl>−Br>−l>−OR>−OH>−NR2>−NH2>−C6H5>−CH=CH2>−H
Recommended topic video on(Inductive Effect)
Solved Examples Based on Inductive Effect
Q.1 Arrangement of (CH3)3C−(CH3)2CH−CH3CH2= when attached to benzyl or an unsaturated group in increasing order of inductive effect is-
(1)(1) $\left(\mathrm{CH}_3\right)_3 \mathrm{C}-<\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-<\mathrm{CH}_3 \mathrm{CH}_2$
(2) $\mathrm{CH}_3 \mathrm{CH}_2-<\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-<\left(\mathrm{CH}_3\right)_3 \mathrm{C}=$
(3) $\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-<\left(\mathrm{CH}_3\right)_3 \mathrm{C}-<\mathrm{CH}_3 \mathrm{CH}_2=$
(4)$\left(\mathrm{CH}_3\right)_3 \mathrm{C}<\mathrm{CH}_3 \mathrm{CH}_2<\left(\mathrm{CH}_3\right)_2 \mathrm{CH}$ (CH3)3C−<(CH3)2CH−<CH3CH2
(CH3)3C<CH3CH2<(CH3)2CH
Solution:
As we learned-
Inductive Effect -When a covalent bond is formed between atoms of different electronegativity, the electron density is more towards the more electronegative atom of the bond. Such a shift of electron density results in a polar covalent bond. Bond polarity leads to various electronic effects in organic compounds. Let us consider chloroethane (CH3CH2Cl) in which the C−Cl bond is a polar covalent bond. It is polarised in such a way that carbon-1 gains some positive charge (δ+) and the chlorine has some negative charge (δ–).
The fractional electronic charges on the two atoms in a polar covalent bond are denoted by the symbol δ (delta) and the shift of electron density is shown by an arrow that points from δ+ to δ– end of the polar bond. In turn, carbon-1, which has developed a partial positive charge (δ+) draws some electron density towards it from the adjacent C-C bond. Consequently, some positive charge (δδ+) develops on carbon-2 also, where δδ+ symbolises a relatively smaller positive charge as compared to that on carbon – 1. In other words, the polar C – Cl bond induces polarity in the adjacent bonds. Such polarization of σ- bond caused by the polarisation of adjacent σ-bond is referred to as the inductive effect.
This effect is passed on to the subsequent bonds also but the effect decreases rapidly as the number of intervening bonds increases and becomes vanishingly small after three bonds. The inductive effect is related to the ability of substituent(s) to either withdraw or donate electron density to the attached carbon atom. Based on this ability, the substituents can be classified as electron-withdrawing or electron-donating groups relative to hydrogen. Electron Withdrawing Groups: Halogens and many other groups such as nitro(−NO2), cyano (−CN), carboxy (−COOH), ester(COOR), aryloxy (-OAr, e.g. −OC6H5), etc. are electron-withdrawing groups. Electron Donating Groups: Alkyl groups like methyl(−CH3) and ethyl (−CH2−CH3)are electron-donating groups.
The greater the number of alkyl groups, the greater the inductive effect.–The CH3 group has a +I effect. As the number of – CH3 group increases the inductive effect increases. Hence, the order of inductive effect is CH3CH2−<(CH3)2CH−<(CH3)3C−
Hence, the answer is the option (2).
Q.2 Select the correct statement about I effect?
(1) I effect transfers electrons from one carbon atom to another.
(2) I effect is the polarization of s bond electrons
(3) I effect creates net charge in the molecule.
(4) I effect is distance-dependent
Solution:
As we learned-
When a covalent bond is formed between atoms of different electronegativity, the electron density is more towards the more electronegative atom of the bond. Such a shift of electron density results in a polar covalent bond. Bond polarity leads to various electronic effects in organic compounds.
Let us consider chloroethane(CH3CH2Cl) in which the C−C bond is a polar covalent bond. It is polarised in such a way that carbon-1 gains some positive charge (δ+)and the chlorine has some negative charge (δ–). The fractional electronic charges on the two atoms in a polar covalent bond are denoted by the symbol δ (delta) and the shift of electron density is shown by an arrow that points fromδ¯+to δ¯ end of the polar bond.
In turn, carbon-1, which has developed a partial positive charge (δ+) draws some electron density towards it from the adjacent C-C bond. Consequently, some positive charge (δδ+) develops on carbon-2 also, where δδ+ symbolizes a relatively smaller positive charge as compared to that on carbon – 1.
In other words, the polar C – Cl bond induces polarity in the adjacent bonds. Such polarization of σ- bond caused by the polarisation of adjacent σ-bond is referred to as the inductive effect. This effect is passed on to the subsequent bonds also but the effect decreases rapidly as the number of intervening bonds increases and becomes vanishingly small after three bonds.
The inductive effect is related to the ability of substituent(s) to either withdraw or donate electron density to the attached carbon atom. Based on this ability, the substituents can be classified as electron-withdrawing or electron-donating groups relative to hydrogen.
(1) Electron Withdrawing Groups: Halogens and many other groups such as nitro(−NO2), cyano (−CN), carboxy (−COOH), ester (COOR), aryloxy (-OAr, e.g. −OC6H5),etc. are electron-withdrawing groups.
(2) Electron Donating Groups: Alkyl groups like methyl (−CH3) and ethyl (−CH2−CH3)are electron-donating groups
The displacement of $\sigma$ electron towards a more electronegative atom is called the inductive effect.
I Effect is distance-dependent.
Hence, the answer is the option (4).
Conclusion
As the name implies, it refers to the situation in which an unbalanced distribution of bonding electrons occurs in any given molecule, causing a permanent dipole to form in the molecule. The formation of permanent polarisation as a result of the partial displacement of sigma e- along the carbon chain or partial displacement of sigma-bonded electron toward more electronegative atom in the carbon chain (i.e., the magnitude of partial positive charge) is referred to as the sigma effect. The inductive effect is a long-term phenomenon. This effect can occur in both sigma and pi bonds, but the electromeric effect can occur only in sigma bonds and pi bonds. The influence on electron density in a region of a molecule caused by electron-withdrawing or electron-donating groups in other portions of the molecule is referred to as electron density shift.
Also Read
11 Mar'25 05:47 PM
19 Feb'25 12:54 PM
18 Feb'25 11:58 PM
18 Feb'25 07:00 PM
18 Feb'25 06:11 PM
18 Feb'25 12:56 PM
18 Feb'25 12:33 PM
19 Oct'24 02:45 PM
19 Oct'24 12:48 PM
19 Oct'24 12:46 PM