Infrared Spectroscopy - Definition, Principle, Hooke’s Law, IR Graph, Application with FAQs
What is Spectroscopy?
Spectroscopy is the branch of science that deals with the study of responses of the molecule when it is exposed to certain kinds of radiations. A given molecule, when exposed to radiation, absorbs a part of it and gets excited and goes to higher energy. The amount and the type of wavelength of radiation absorbed by the molecule in order to reach the excited state depends upon the structural features of the molecule. Therefore, by studying the type of radiation absorbed, it is possible to predict what kind of structural features are present in the molecule.
The absorption of different types of radiations such as UV, visible, infrared, microwaves, radio waves produce different kinds of excitations of the molecule and each excitation provides some important information about the structure. Since in all these methods, the nature of the radiation absorbed is studied, the spectroscopic methods are classified as “absorption spectroscopy”
IR Spectroscopy
IR spectroscopy is Infrared spectroscopy. IR spectroscopy of an organic compound reveals a good deal of information about the functional groups present in the compound. IR spectroscopy slideshare can be helpful in understanding the concept.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
IR Spectrum
In IR spectroscopy, the molecule is exposed to infrared radiations and the responses given by the molecule are recorded, generally in the form of a graph called the IR spectrum.
Principle of IR spectroscopy
Atoms in any molecule and molecule as a whole are never stationary. They continuously rotate, vibrate and also move from one point to another point. Even at 0 K, when the kinetic energy of the entire molecule is zero, the atomic nuclei vibrate about the bonds which connect them.
The atoms in the molecule can vibrate in a number of ways. Each vibration requires a definite amount of energy, that is the molecule has a number of vibrational energy levels, each of which is quantised. If a molecule is exposed to IR radiations, the molecules absorb the energy of the IR radiation and as result, gets excited to higher vibrational energy levels. The type of the IR wavelengths absorbed by the molecule depends on the type of the atoms and the chemical bonds in the molecule. When these absorptions are recorded, we get the IR spectrum.
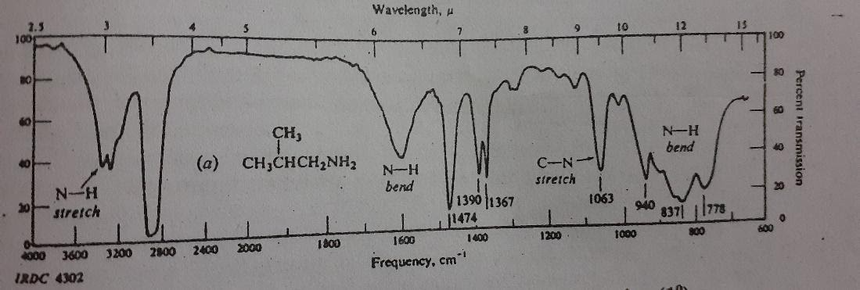
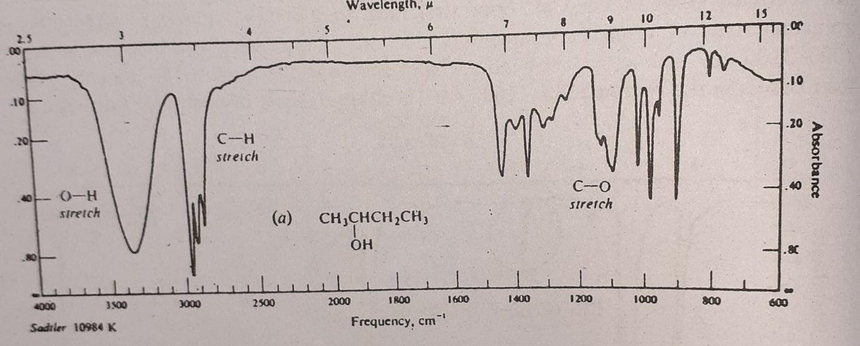 A typical IR spectrum
A typical IR spectrum
IR region
The whole IR region is subdivided into three parts
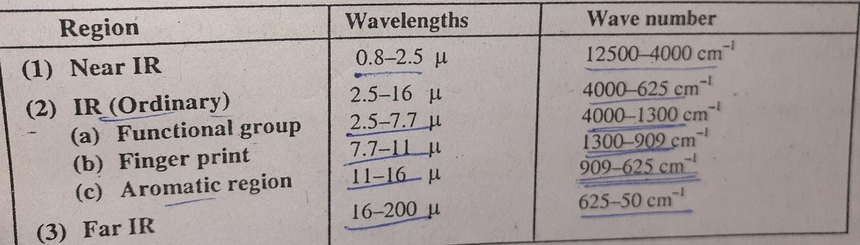
The region between 2.5 to 16µ is very useful from the view of organic chemists because most of the fundamental vibrations of the molecule occur in this region.
- The common functional groups show absorption bands in the region 2.5 to 7.7µ. This region is known as the functional group region. The bands that occur in this region are due to stretching vibrations of the functional groups in the molecule.
- The region between 7.7 to 11µ is known as fingerprint region and is very complex and is a characteristic for a specific molecule. The bonds that appear in this region are due to skeletal vibrations of C-C, C-O, C-N bonds in the molecule. The region is very useful for sample comparison. If the fingerprint region of two samples are identical, then these samples are also identical.
- The region between 11 to 16µ is known as the aromatic region. The band that occurs in this region is due to bending vibrations of C-H bonds in aromatic compounds.
Hooke’s law in IR spectroscopy
The vibrational frequency of a bond may be related to the masses of vibrating atoms and the force constant (f) of the vibrating bond. This corresponds to a simple Hooke’s law model of two units connected by a spring in which the force constant is the restoring force provided by the spring.
IR spectroscopy instrumentation
The IR spectroscopy instrumentation is shown below.
Initially, a ray of IR radiation is passed through the reference from the source into the sample. The two beams are then reflected to pass first through a splitter and then second to a detector.
And lastly, the needed reading is taken out through printing out after the processor deciphers the passed data into the detector.
Related Topics Link, |
IR working
Infrared spectroscopy requires infrared rays as a source of radiation to excite the compound molecules, generating a spectrum of infrared of the absorbed energy by a molecule as a function of the wavelength or frequency of light.
The important fact to know is that a dipole moment is needed for a molecule to absorb IR radiation. Basically, a bond with a dipole moment with stretching frequency causes IR spectrum absorption.
IR graph
Sample handling in IR spectroscopy
In IR spectroscopy three types of samples can be used that are gaseous, liquid and solid types.
- Samples in liquid state are transferred between two plates of salt which are measured because they are transparent in presence of IR light. Potassium bromide, sodium chloride and calcium fluoride are generally used in making salt plates.
- The samples of gaseous state should have long pathlength because gaseous state samples are in ppm concentration so that the light should travel, generally for a long distance into the sample cell.
- Solid state samples are made by crushing the substance with an agent which has an oily layer.
FTIR spectroscopy
FTIR spectroscopy is also known as Fourier-Transform infrared. The FTIR principle is based on infrared spectroscopy in which an IR spectrum is obtained through an absorption of infrared samples which in continuation collects high resolution data spectrum over a long range of spectral networks.
Also, students can refer,
Application of IR spectroscopy
- Determination of structure of molecules
- Functional group region: the observation of peaks in the functional group region will help us to understand the functional groups present in the molecule. If peaks are shifted to higher or lower values than the expected values, indicates the presence of either inductive or resonance or steric effects present in the molecule.
- Fingerprint region: the peaks in this region are very characteristic for the molecule. No two different molecules can have identical IR spectra in this region and if they have then they are identical in nature. The comparison of the IR spectrum in the fingerprint region helps us to establish the identity of the molecule.
- Aromatic region: the C-H bending vibrations in this region help us to know the substitution pattern in aromatic compounds.
- Study of progress of chemical changes: IR spectroscopy is often used to follow the progress of chemical reaction, to control the chemical reaction, and to study the kinetics of various chemical reactions. These applications are based on the fact that most chemical reactions involve changes in the functional groups. This helps us to follow up a course of chemical reaction. A sample of the reaction mixtures is withdrawn and IR spectrum is recorded. The spectrum is used to determine how far the reaction has progressed and whether it is proceeding by the expected path or not.
- Detection of hydrogen bonding: IR spectroscopy distinguishes between intramolecular “H” bonding, intermolecular “H” bonding and free -OH group. Free-OH group appears at higher frequency while “H” bonded alcohols and phenol appear at lower frequency.
- Determination of size of ring ketones: Ring strain in cyclic ketones shifts the >C=O stretching frequency to a higher wavelength. As ring strain increases, >C=O frequency also increases. This enables us to differentiate the ring size of ketones.
- Detection of impurity: we know that a particular functional group absorbs at particular frequency, known as group frequency. If the hydrocarbons contain a very small amount of ketone can be easily traced out by IR spectrum. This is because a ketone shows a strong absorption band at 1720 cm-1, while hydrocarbons do not have an absorption band at 1720 cm-1. Similarly, a small quantity of alcohol in hydrocarbon mixture can be easily detected by observing a broad absorption band at 3200-3600 cm-1 due to OH stretching frequency, which is absent in hydrocarbons.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: