Ionic Bond - Partially Covalent in Nature
If the atoms that form a covalent bond are identical, as in H2, Cl2, and other diatomic molecules, then the electrons in the bond must be shared equally. We refer to this as a pure covalent bond. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus.
In the case of Cl2
Each atom starts with seven valence electrons, and each Cl shares one electron with the other, forming one covalent bond:
Cl+Cl⟶Cl2
When the atoms linked by a covalent bond are different, the bonding electrons are shared, but no longer equally. Instead, the bonding electrons are more attracted to one atom than the other, giving rise to a shift of electron density toward that atom. This unequal distribution of electrons is known as a polar covalent bond, characterized by a partial positive charge on one atom and a partial negative charge on the other. The atom that attracts the electrons more strongly acquires the partial negative charge and vice versa.
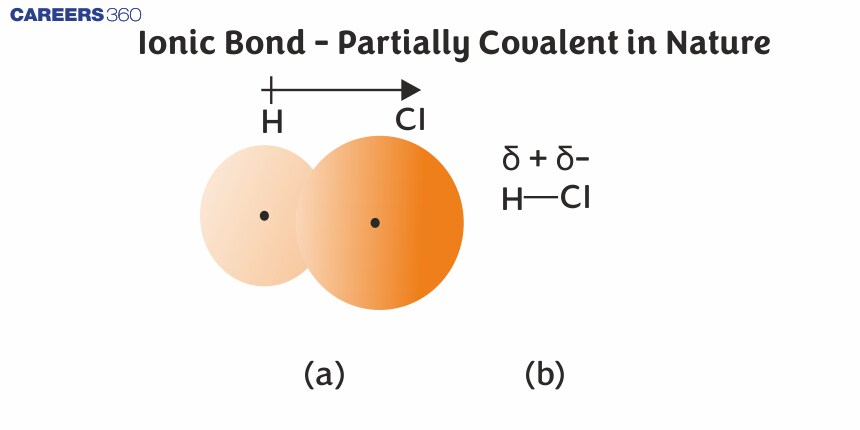
For example, the electrons in the H–Cl bond of a hydrogen chloride molecule are shifted towards chlorine. Thus, in an HCl molecule, the chlorine atom carries a partial negative charge and the hydrogen atom has a partial positive charge as shown in the figure given below.

(a) The distribution of electron density in the HCl molecule is uneven. The electron density is greater around the chlorine nucleus. (b) Symbols δ+ and δ– indicate the polarity of the H–Cl bond.
When the electronegativity difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent, or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H–H, H–Cl, and Na–Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). The figure below shows the relationship between electronegativity difference and bond type.

As the electronegativity difference increases between two atoms, the bond becomes more ionic.
Recommended topic video on (Ionic character in covalent bond)
Some Solved Examples
Example 1: The values of electronegativity of atoms A and B are 1.80 and 4.0 respectively. The percentage of ionic character of the A-B bond is?
1)43.14%
2)50 %
3)55.3 %
4) 52.14 %
Solution
As we learn
Percent ionic character = 16 (XB-XA) + 3.5 (XB-XA)2
Where XB is the electronegativity of atom B and XA is the electronegativity of A
So, Percent ionic character = 16 (4-1.8) + 3.5 (4-1.8)2
= 16 X (2.2) + 3.5 X (2.2)2
= 35.2 + 16.94
= 52.14
Hence, the answer is the option (4).
Example 2: The electronegativities of F, Cl, BrF,Cl, Br and II are 4.0, 3.0, 2.8 4.0,3.0,2.8 and 2.5 respectively.Hydrogen halide with a high percentage of ionic character is
1)HFHF
2) HCl
3) HBr
4) HI
Solution
Ionic Character in Covalent Bond -
When the electronegativity difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H–H, H–Cl, and Na–Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). The figure below shows the relationship between electronegativity difference and bond type.
As the electronegativity difference increases between two atoms, the bond becomes more ionic.
The greater the electronegativity difference more is the ionic character. Thus, H-F has maximum ionic character.
Hence, option number (1) is correct.
Example 3: If the molecule of HCl were polar, the expected value of dipole moment is 6.12 D(debye) but the experimental value of dipole moment was 1.03D. The percentage ionic character is:
1) 17
2)83
3)50
4)Zero
Solution
The percentage ionic character is given by the following formula
$\%$ Ionic Character $=\frac{\text { experimental value of dipole moment }}{\text { theoretical value of dipole moment }} \times 100=\frac{1.03 \mathrm{D}}{6.12 \mathrm{D}} \times 100=16.83 \%=17 \%$ Hence, the answer is the option (1).
Example 4: Bond distance in HF is 9.17×10−11m′ 9.17×10−11m′ Dipole moment of HF is 6.104×10−30 cm2 6.104×10−30 cm2The percent ionic character in HF will be :
(electron charge ==1.60×10−19C)=1.60×10−19C))
1)61.1%
2)38.0%
3)35.5%
4)41.5%
Solution
$\begin{aligned} & \text { Given, } e=1.60 \times 10^{-19} \mathrm{C}, \mathrm{d}=9.17 \times 10^{-11} \mathrm{~m} \text { From } \\ & \quad \mu=e \times d \mu=1.60 \times 10^{-19} \times 9.17 \times 10^{-11}=14.672 \times 10^{-30} \\ & \% \text { ionic character }=\frac{\text { Observed dipole moment }}{\text { Dipole moment for 100 }}=41.5 \%\end{aligned}$
Hence, the answer is the option (4).
Example 5: Which of the following is the most ionic?
1)P4O10
2) MnO
3)CrO3
4)Mn2O7
Solution
Magnitude of positive charge α polarization power
α covalent character
α 1/ ionic character
If the magnitude positive charge is high then it will be very unstable to remain as an ion because it needs an electron to get stable that's why it makes a covalent bond at a high magnitude positive charge. magnitude positive charge
P4O10 P = +5
MnO Mn = +2
CrO3 Cr = + 6
Mn2O7 Mn = + 7
Among them, MnO has the lowest magnitude of positive charge. Hence, MnO is the most ionic compound.
Hence, the answer is the option (2).
Summary
When the bond is formed between two positively charged atoms is called ionic bond the force that joined these bonds is the electrostatic force of attraction.