Law of Equivalence: Definition, Formula, Questions and Examples
The equivalents of all substances that react or are produced are always equal in a chemical reaction, this is what Law of Equivalence teaches. How much a substance can react—measured using its weight divided by its equivalent mass, or via normality and volume is known to be an equivalent. This rule works in situations like acid–base neutralizations and redox titrations, where we can rely on the fact that the number of equivalents of acids and bases (or oxidants and reductants) must balance, even if the chemical equation isn’t fully balanced.
This Story also Contains
- Laws of Equivalence
- Types of Equivalents
- Application in Real Life and its Implication
- Some Solved Examples
- Conclusion
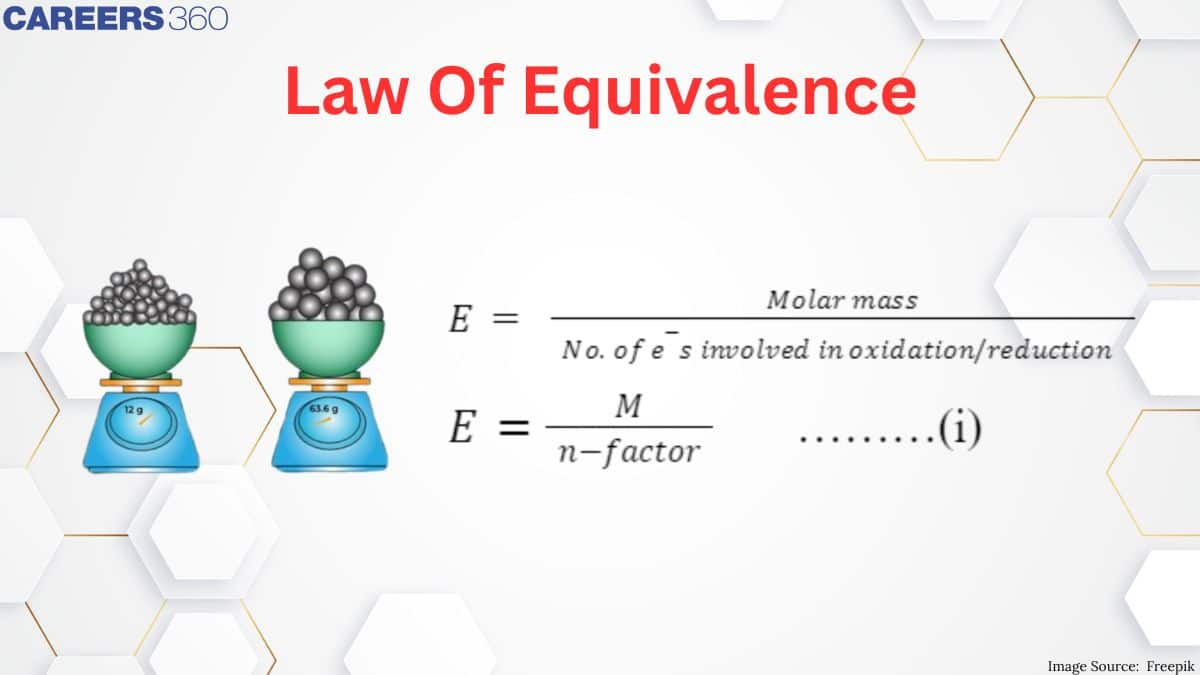
Laws of Equivalence
According to the law of equivalence, for each and every reactant and product,
Equivalents of each reactant reacted = Equivalents of each product formed.
Example,
Suppose the reaction is taking place as follows:
$P+Q \rightarrow R+S$
According to the law of equivalence,
Equivalents of P reacted = Equivalents of Q reacted = Equivalents of R produced = Equivalents of S produced
Equivalents of any substance = (Weight of substance (in g)) / (Equivalent weight)
= Normality (N) x Volume (V) (In liter)
Normality (N) = n-Factor x Molarity (M)
Law of Equivalence finds great importance in Acid-base Neutralisation Reactions as well as Redox Titrations.
Here we shall be mainly covering the Acid-Base neutralization reactions in detail.
Types of Equivalents
1. General Equivalent:
An equivalent is the amount of a substance that reacts with or is equivalent to one mole of another in a specific reaction—such as supplying one mole of H⁺ ions in acid-base reactions or one mole of electrons in redox reactions.
2. Acid–Base Equivalents:
-
For acids an equivalent is based on how many H⁺ ions the acid can donate.
-
For bases, it's the number of OH⁻ ions the base can supply.
For example, 1 mole of H₂SO₄ can donate 2 H⁺ ions—thus it’s equivalent to 2 equivalents.
3. Redox Equivalents:
In redox reactions, equivalents refers to how many electrons are transferred. Forexample, in acidic solutions, one mole of KMnO₄ (Mn from +7 to +2) corresponds to 5 equivalents because it accepts 5 electrons.
$\mathrm{MnO}_4^{-}+5 \mathrm{Fe}^{2+}+8 \mathrm{H}^{+} \rightarrow \mathrm{Mn}^{2+}+5 \mathrm{Fe}^{3+}+4 \mathrm{H}_2 \mathrm{O}$
4. Equivalent Weight (Equivalent Mass):
This is the mass of a substance that corresponds to one equivalent.
-
Calculated by dividing molecular weight by the valency factor (n-factor)
5. Electrochemical Equivalent :
Used in electrochemistry, this measures the mass of a substance deposited or liberated per unit of electricity—typically grams per coulomb. Values vary by element (e.g., copper, silver, etc.)
6. Normality (Equivalent Concentration):
Normality (N) is the number of equivalents per liter of solution.
-
In acid-base chemistry, it reflects equivalents of H⁺ or OH⁻.
-
In redox reactions, it corresponds to electrons transferred.
Application in Real Life and its Implication
The law of equivalence is very essential in practical chemistry. In titrations, it confirms accurate measurements by comparing reactive equivalents of acids and bases. It is also helpful in redox reactions and volumetric analysis, empowering precise calculations of reactants and products. Industries depends on it for tasks such as water treatment—monitoring chlorine or oxygen levels—and food and pharmaceutical testing, such as measuring vitamin C or iron content. The principle bridges theoretical stoichiometry with real lab and industrial practices, enhancing reliability, safety, and efficiency in chemical processes.
Also Read:
Recommended topic video on(Law of Equivalence)
Some Solved Examples
Example 1:In this equation:
What will be the mass (in g) of O2 produced from 1.23g of KClO3?
1) 0.482
2) 0.545
3) 0.758
4) 0.345
Solution:
According to the equation,
2 moles of KClO3 will produce 3 moles of O2.
Thus moles of KClO3 = 1.23 / 123= 0.01 moles
Therefore, 0.01 moles of KClO3 will produce = (3 x 0.01) / 2= 0.015 moles
thus mass of O2 = 0.015 x 32
= 0.48g
Hence, the answer is the option (1).
Example 2:
50 mL of 0.5 M oxalic acid is needed to neutralize 25 mL of sodium hydroxide solution. What is the mass (in g) of NaOH in 50 mL of the given sodium hydroxide solution?
1) 20
2) 4
3) 10
4) 40
Solution
$\begin{aligned} & \mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4+2 \mathrm{NaOH} \rightarrow \mathrm{Na}_2 \mathrm{C}_2 \mathrm{O}_4+2 \mathrm{H}_2 \mathrm{O} \\ & \text { meq of } \mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4=\text { meq } \mathrm{NaOH} \\ & 50 \times 0.5 \times 2=25 \mathrm{MNaOH} \times 1 \\ & 1000 \mathrm{ml} \text { solution }=2 \times 240 \text { gramNaOH } \\ & \therefore 50 \mathrm{ml} \text { sol }=4 \mathrm{gNaOH}\end{aligned}$
Hence, the answer is the option (2).
Example 3:
The volume of 0.1 N dibasic acid sufficient to neutralize 1 g of a base that furnishes 0.04 mol of OH− in aqueous solution is:
1) 200mL
2) 400mL
3) 600mL
4) 800mL
Solution
Dibasic acids mean acids have two ionizable hydrogens.
gram eq. of $\mathrm{OH}^{-}=$mole $\times$n-factor $=0.04 \times 1=0.04$
The Law of equivalence we get,
milli equivalents of acid = milli equivalents of base
N1 X V1 (ACID) = ( meq. of base)
0.1×V = ( meq. of base)
0.1 X V = ( meq. of base) = 0.04$ \times 1000 $
V x 0.1 = 40
V = 400ml
Hence, the answer is an option (2).
Example 4:
How much NaOH is required to neutralize 1500 mL of 0.1 M HCl?
1) 40g
2) 4g
3) 6g
4) 60g
Solution
According to the law of Equivalence,
meq. of HCl = meq. of NaOH
moles of $\mathrm{NaOH}=150$
Weight of $\mathrm{NaOH}=6 \mathrm{~g}$
Hence, the answer is the option (3).
Example 5:
To neutralize 20 mL of M10 sodium hydroxide, the volume of M20 hydrochloric acid required is:
1) 10ml
2)15ml
3) 20ml
4) 40ml
Solution
According to the law of equivalence,
meq. of HCl = meq. of NaOH
$\begin{aligned} & \therefore 20 \times \frac{1}{10}=\times \frac{1}{20} \\ & =40 \mathrm{ml}\end{aligned}$
Hence, the answer is the option (4).
Practice more Questions From the Link Given Below:
Conclusion
The Law of Equivalence expresses that in any chemical reaction, the number of equivalents of reactants is always equal to the number of equivalents of products. It makes it easy to perform calculations in titrations and redox reactions, even without a balanced equations. Law of equivalence links chemistry theory to practical use, enhancing accuracy, safety, and efficiency in laboratory and industrial processes by ensuring proper reactant usage and reliable outcomes.