Metals, Non-metals and Metalloids
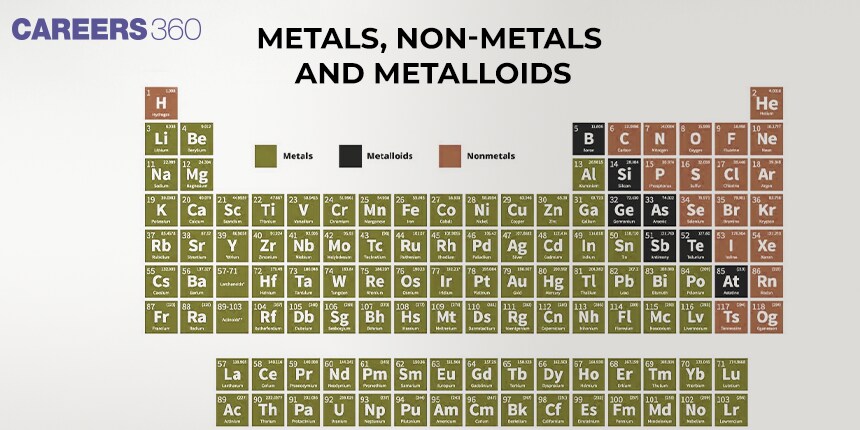
Elements in chemistry are commonly grouped into a metals group, non-metals group, and the metalloids group with classification based on the elements' physical and chemical characteristics. Elements on the left side and middle of the periodic table are generally metals. Elements that are located on the right-hand side of the periodic table are referred to as non-metals and do not exhibit characteristics similar to those of the metals. Metalloids, which are positioned on the table between metals and non-metals as a diagonal, have the features of both.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- From Conductivity to Brittleness: Metal, Non-Metal and Metalloids
- Metals
- Non-metals
- Metalloids
- Some Solved Examples
- Conclusion
In this article, we will cover the concept of Metal, Non-metal and Metalloids in chemistry. This concept falls under the broader category of Classification of elements, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more. Over the last ten years of the NEET and JEE exams (from 2013 to 2023), one question in JEE in 2020 has been asked on this concept.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
From Conductivity to Brittleness: Metal, Non-Metal and Metalloids
The elements can be divided into three categories i.e., metals and non-metals and metalloids. Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table. Metals are usually solids at room temperature except mercury which is liquid at room temperature and even gallium and caesium also have very low melting points (303K and 302K. respectively). Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable and ductile.
Metals
The vast majority of elements in the periodic table of elements are identified as metals. The metals are positioned on the periodic table to the left of the zigzag line that connects the tellurium, astatine, silicon, boron, and arsenic elements. Metalloids and semimetals can be used for elements that fall on or slightly to the left of the line: boron, silicon, germanium, arsenic, tellurium, antimony, and polonium. This indicates that they possess characteristics of both nonmetals and metals.
Non-metals
are placed at the top right side of the Periodic Table. In a period, the properties of elements change from metallic on the left to non-metallic on the right. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). They are poor conductors of heat and electricity. Most non-metallic solids are brittle and are neither malleable nor ductile. The elements become more metallic as we move from top to bottom in a group and the non-metallic character increases as we move from left to right in a period.
Metalloids
There are some elements whose properties are in between the metals and non-metals. They are known as metalloids. There are a total of 8 metalloids in the periodic table i.e, boron(B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At).
Recommended topic video on(Metals, non-metals and Metalloids)
Some Solved Examples
Example 1: Generally, metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
1) H2SO4
2) HCl
3) (correct) HNO3
4) All of these
Solution: Classification of Elements as Metals, Non-metals and Metalloids -
The elements can be divided into three categories, i.e., metals, non-metals, and metalloids. Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table. Metals are usually solids at room temperature except for mercury, which is liquid at room temperature, and even gallium and caesium also have very low melting points (303K and 302K., respectively). Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable and ductile.
Nitric acid(HNO3) is a strong oxidising agent. The Hydrogen gas produced during its reaction with metal gets oxidised to H2O, hence no hydrogen gas is produced.
Hence, the answer is the option (3).
Example 2: The lightest metal in the periodic table is :
1) Ca
2) Hg
3) Na
4) (correct) Li
Solution: Metals -
– Form metallic bonds.
– 78% of all known elements.
– good conductor of heat & electricity.
– Malleable and ductile.
Li is the lightest metal in the periodic table.
Hence, the answer is the option (4).
Example 3: Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): Metallic character decreases and non-metallic character increases on moving from left to right in a period.
Reason (R): It is due to an increase in ionisation enthalpy and a decrease in electron gain enthalpy, when one moves from left to right in a period.
In the light of the above statements, choose the most appropriate answer from the options given below :
1) (A) is false but (R) is true.
2) Both (A) and (R) are correct and (R) is the correct explanation of (A).
3) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
4) (correct) (A) is true but (R) is false.
Solution: On moving from left to right in a period, the metallic character decreases while the non-metallic character increases. This is due to an increase in Ionisation enthalpy and an increase in the magnitude of electron gain enthalpy.
Thus, Assertion (A) is true while Reason (R) is false
Hence, the answer is the option (4).
Example 4: The lustre of a metal is due to
1) It's high polishing
2) Its high-density
3) Chemical inertness
4) (correct) Presence of free electrons
Solution: As we learned in, Metallic solids -
Metallic bond, i.e., the attraction between the positively charged metal ion and mobile electron.
Ex. iron, copper, zinc, aluminium, sodium
The metallic lustre of a metal is due to the presence of free electrons.
Hence, the answer is the option (4).
Example 5: Which is the only metal that exists in liquid form at room temperature?
1) Br2
2) Ag
3) W
4) (correct) Hg
Solution: Hg as mercury exists as a liquid at room temperature.
Hence, the answer is an option (4).
Example 6: Which one of the elements is a non-metal?
1) (correct) C
2) Al
3) Na
4)Cr
Solution: Non- Metal -
– Located at the right of the Periodic Table
– Poor conductor of heat & electricity
– They are usually solids or gases at room temperature.
C is a non-metal
Hence, the answer is the option (1).
Example 7: Given below are two statements:
Statement I: Both metals and non-metals exist in p and d-block elements.
Statement II: Non-metals have higher ionization enthalpy and higher electronegativity than the metals.
In the light of the above statements, choose the correct answer from the options given below:
1) Both statement I and statement II are false
2)Both statement I and statement II are true
3) (correct) Statement I is false but statement II is true
4)Statement I is true but statement II is false
Solution:
Statement I: p-block has Metal as well non metals. while d-block has only metal. hence Ist is incorrect. Statement II : Non-Metal has high I.E. & E.N.
F→ highest E.N.
He→ Highest I.E.
Hence, the answer is the option (3).
Example 8: Which of the following elements is considered a metalloid?
1) Sc
2) Pb
3) Bi
4) (correct) Te
Solution: Fact Based.
Sc,Pb and Bi are metals while Te is a metalloid.
Hence, the answer is the option (4).
Example 9: Which of the elements are metalloids?
1) B
2) Si
3) Sb
4) (correct) All of these
Solution: Metalloids or Semi-metals -
They show the properties of both metals and non-metals. Their change from metallic to non-metallic character is non-abrupt.
B, Si, and Sb all are metalloids.
Hence, the answer is the option (4).
Conclusion
Therefore, the division of elements in terms of metals, non-metals, and metalloids gives a primary understanding of the various parts of these aspects of chemistry. Some metals have shining surfaces, electrical conductivity and ductility, and they are used in manufacturing industries, structuring and electrical industries as well. Some of the main applications of non-metals which are described as brittle and poor conductors of heat and electricity include roles in biosystems, for environmental applications, and as the constituent of different compounds and materials. Metalloids; chemical elements displaying characteristics of both metals and non-metals find critical utilization in the technology of semiconductors which culminated in the state-of-the-art technological inventions in electronics and computers.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
Frequently Asked Questions (FAQs)
Mercury is the only metal which liquid at room temperature.
Non-metals are commonly solid or gaseous at room temperature and have low melting and boiling points.
Metalloids are commonly used in semiconductors, alloys and biological agents.
Metalloids are commonly used in semiconductors, alloys and biological agents.
Palladium is amongst the four most expensive metals besides Gold, Silver and Rhodium.
Also Read
12 Mar'25 09:34 AM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
09 Jan'25 03:51 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM