Methods of Preparation of Aldehydes and Ketones
One enters a bakery and is greeted by the smell of newly baked bread and cookies. These are compounds with a pleasing odor known as aldehydes and ketones. Aldehydes and ketones fall under the category of such important organic compounds that find an application in foodstuffs industries, pharmaceuticals, and perfumes. From the smell of freshly cut grass—sharp and crisp, due to an aldehyde—to the sharp, sweet nail polish remover smell, a ketone, these chemicals enrich our senses.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- General Concept and Definitions
- Preparation of Aldehydes
- Preparation of Ketones
- Relevance and Applications
- Rosenmund Reduction
- Stephen Reduction
- Some Solved Examples
- Summary
Aldehydes and ketones do not restrict themselves to the sensory arena of pleasures; on the other hand, they turn out to be some important classes of intermediates for the synthesis of many pharmaceuticals and fine chemicals. Chemists and industrialists dealing with these versatile molecules should be conversant with how to prepare such compounds. In this paper, we will take a closer look at the methods of preparation of aldehydes and ketones and their significance or applications.
General Concept and Definitions
Aldehydes and ketones are a class of organic compounds that contain a carbonyl group,$\mathrm{C}=\mathrm{O}$. In aldehydes, the carbonyl group is bonded to at least one hydrogen atom and is always located at the end of the chain. Familiar examples include formaldehyde and benzaldehyde. In the case of the ketones, the carbonyl group is bonded to two carbon atoms, thus being part of the carbon chain. Acetone and butanone are familiar ketones. These are of great importance as compounds in organic chemistry because of their reactivity and the wide range of reactions they undergo. Knowing their methods of preparation provides a basis for their further study of chemical behavior and applications.
Preparation of Aldehydes
There are many ways to make aldehydes. All of them require a particular set of chemical circumstances to best yield the desired product. One common method is simply the oxidation of primary alcohols. For instance, ethanol can be oxidized to acetaldehyde with a mild oxidizing agent such as PCC (Pyridinium chlorochromate). Alkenes can be transformed into aldehydes by hydroformylation, where an alkene reacts with carbon monoxide and hydrogen in the presence of a catalyst. Reduction of acid chlorides by reagents like lithium tri-tert-butoxyaluminum hydride also forms aldehydes. These two techniques bring versatility to the preparation of aldehydes for various uses.
Oxidation
Oxidation of ROH involves the cleavage of (O-H) and (C-H) bonds to form (C=O) bonds. Such reactions are also called dehydrogenation reactions because H2 is lost from an alcohol molecule. Strong oxidizing agents such as acidic $\mathrm{KMnO}_4$ or acidic $\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7$ oxidize 1o ROH first to an aldehyde and then to carboxylic acids. Further, 2o alcohols are oxidized to ketones and 3o alcohols do not undergo oxidation, but under drastic conditions such as strong oxidizing agents(KMnO4) and at high temperatures cleavage of (C-C) bonds takes place and a mixture of RCOOH containing a lesser number of C atoms is formed. 1o ROH can be converted up to the aldehyde stage either by the use of ${CrO}_3$ in an anhydrous medium or with PCC or with Jones reagent.
Some examples include:
$\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_2 \mathrm{OH} \xrightarrow{\mathrm{PCC}} \mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CHO}$
Dehydrogenation
1o alcohols undergo dehydrogenation to give aldehydes only. 2o alcohols give ketones and 3o alcohols give alkenes. Some examples include.
![]()

Preparation of Ketones
Methods of preparation of ketones are mainly from the oxidation of secondary alcohols. For example, isopropanol gets oxidized to acetone using an oxidizing agent like chromic acid or potassium permanganate. The other way is through Friedel-Crafts acylation in which an aromatic compound is caused to react with an acyl chloride under a Lewis acid catalyst to produce a ketone. Ketones can also be prepared by hydration of alkynes, essentially meaning that an alkyne reacts with water in the presence of a catalyst, such as acid. Each one permits tailoring of the synthesis process according to specific needs and conditions.
Relevance and Applications
The preparation of aldehydes and ketones is not an exercise in methodology; it has a deep practical impact. Many drugs are aldehydes or ketones, and effective routes for their synthesis are in demand from the pharmaceutical industry. One example is the anti-inflammatory pain reliever ibuprofen, whose synthesis involves ketone intermediates. In foods, aldehydes give the flavors of vanilla, cinnamon, and many other essential oils that add richness to food experiences. In perfumery, 5%-25% concentrations of aldehydes and ketones in perfumes give them their appeal and lasting qualities. With their diversified methods of preparation, better processes and products can be designed that have impacts on several aspects of everyday life.
Rosenmund Reduction
Partial hydrogenation of benzoyl chloride with finely divided Pd as a catalyst in the presence of $\mathrm{BaSO}_4$ and S or quinoline in boiling xylene as solvent gives benzaldehyde. This reaction is called Rosenmund reduction. The catalyst under the above condition is called Lindlar's catalyst or poisoned Pd. The Lindlar's catalyst also reduces $(\mathrm{C}=\mathrm{C})$ to $(\mathrm{C}=\mathrm{C})$in syn-addition.
It is BaSO4 that prevents the aldehyde from being further reduced to alcohol and acts as a poison to the Pd catalyst. A small amount of sulfur and quinoline is very effective in poisoning the catalyst in aldehyde reduction. Moreover, S and quinoline react with a small amount of $\mathrm{H}_2$ to give $\mathrm{H}_2 \mathrm{~S}$ gas and hydroquinoline, thereby limiting H2 for further reduction of aldehyde to alcohol.
The reaction occurs as follows:

Stephen Reduction
Nitriles are partially reduced to corresponding imine with SnCl2 in the presence of HCl, which on hydrolysis gives the corresponding aldehyde. It does not reduce$(\mathrm{C}=\mathrm{C})$ or $(\mathrm{C}=\mathrm{C})$. This reaction is known as Stephen reduction. The reaction occurs as follows.

From Acyl chlorides
Formyl chloride gives an aldehyde and all other halides five ketones. The reaction occurs as follows.
![]()
From Nitriles
Grignard reagents give aldehydes with hydrogen cyanide (HCN) and ketones with alkyl cyanides (RCN). The reaction occurs as follows:

Recommended topic video on(Methods of Preparation of Aldehydes and Ketones)
Some Solved Examples
Example 1
Question:
Ethanol upon treatment with which one of the following will give ethanal?
$
\begin{aligned}
& \text { 1) } \mathrm{KMnO}_4 / \mathrm{H}_2 \mathrm{SO}_4 \\
& \text { 2) } \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}_2 \mathrm{SO}_4
\end{aligned}
$
3) PCC
$
\text { 4) } \mathrm{KMnO}_4 / \mathrm{NH}_4 \mathrm{Cl}
$
Solution:
As we learned
PCC is a mild oxidizing agent and it is used to convert primary or secondary alcohols into aldehydes and ketones respectively. 
Therefore, Option(3) is correct
Example 2
Question:
Propan-2-ol upon treatment with which reagent will give acetone?
$\begin{aligned} & \mathrm{KMnO}_4 / \mathrm{H}_2 \mathrm{SO}_4 \\ & \mathrm{PDC} \\ & \mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7 / \mathrm{H}_2 \mathrm{SO}_4 \\ & \mathrm{KMnO}_4 / \mathrm{NH}_4 \mathrm{Cl}\end{aligned}$
Solution:

Therefore, Option(2) is correct
Example 3
Question:
$\mathrm{R}-\mathrm{CN} \xrightarrow{\mathrm{SnCl}_2 / \mathrm{HCl}} A \xrightarrow{\mathrm{H}_2 \mathrm{O}} B+C$
B is :
1)RCONH2
2)RCHO
3)RNC
4)RCOOH
Solution:
As we have learned
Stephen's reduction -
By Stephen's reduction cyanides are converted into imines which on hydrolysis convert into aldehydes or ketones.
- wherein
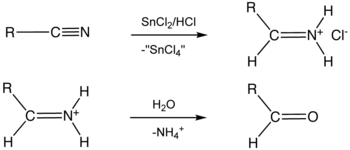
$\mathrm{R}-\mathrm{C} \equiv \mathrm{N} \underset{2[\mathrm{H}}{\mathrm{l}} \mathrm{SnCl}_2 / \mathrm{HClRCH}=\mathrm{NH} \cdot \mathrm{HCl} \xrightarrow{\mathrm{H}_2 \mathrm{O}} \mathrm{RCHO}+\mathrm{NH}_4 \mathrm{Cl}$
Summary
There is a summary of the major points regarding aldehydes and ketones, important organic compounds applied in various aspects. We took into consideration their definitions and the general properties of their carbonyl groups. Common ways of preparing aldehydes include the oxidation of primary alcohols, hydroformylation of alkenes, and reduction of acid chlorides. On the other hand, some ways ketones are prepared include oxidation of secondary alcohols, Friedel-Crafts acylation, and hydration of alkynes.
Also Read
27 Nov'24 05:52 PM
18 Oct'24 12:43 PM
18 Oct'24 12:34 PM
18 Oct'24 12:31 PM
18 Oct'24 12:27 PM
18 Oct'24 12:19 PM
18 Oct'24 12:14 PM
18 Oct'24 12:09 PM