Sodium Carbonate (Na2CO3) - Uses, Formula, Properties, FAQs
What is sodium carbonate?
Sodium carbonate, is an inorganic chemical with the formula Na2CO3 and its numerous hydrates (also known as washing soda, soda ash uses, and soda crystals). All of the forms are water-soluble, colourless, odourless salts that produce moderately alkaline solutions in water. It was traditionally derived from the ashes of plants that grew in sodium-rich soils.
The molecular formula of sodium carbonate is Na2CO3. Similarly, Soda ash chemical formula is Na2CO3.10H2O.Because the ashes of these sodium-rich plants differed markedly from those of wood (which were originally used to make potash), sodium carbonate was dubbed "soda ash." The Solvay technique produces it in vast quantities from sodium chloride and limestone. Sodium carbonate is also known as washing soda.
What is Na2CO3?
Sodium carbonate is a chemical compound that is made up of inorganic elements. Soda ash is sodium carbonate, sometimes known as sodium carbonate. Trona is used to remove soda ash. Trona is a double salt made up of sodium carbonate and sodium hydrogen carbonate that forms as a result of evaporation processes in lakes. The most significant of the basic heavy chemicals is sodium carbonate, sometimes known as washing soda or soda ash. It has the advantage of being non-corrosive and so safer to handle than sodium hydroxide.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Sodium Carbonate Formula
Sodium carbonate is a carbonic acid diazonium salt with the chemical formula Na2CO3. Soda crystals, soda ash, and washing soda are some other names for it. This inorganic compound is water-soluble, forming carbonic acid and sodium hydroxide when dissolved in water. It is a white powder with no odour in its purest form. It's a powerful base that also functions as an antacid.
There are four ways to make sodium carbonate: the Solvay process, Labnac process, Dual-process, and Electrolytic process.
It is slightly soluble in ethanol and insoluble in alcohol because it is a weak acid. One of the most common applications for Na2CO3 is as a water softener. The pH level is approximately 11.
Hydrates of Na2CO3
Three hydrates and the anhydrous salt of sodium carbonate are available:
Na2CO3.10H2O, sodium carbonate decahydrate (natron), readily effloresces to form the monohydrate.
Na2CO3.7H2O, sodium carbonate heptahydrate (not known in crystalline form).
Na2CO3.H2O, sodium carbonate monohydrate (thermonatrite). Crystal carbonate is another name for it.
Na2CO3 Calcined soda, also known as anhydrous sodium carbonate. The hydrates are heated to create it. It can also be generated when sodium hydrogen carbonate is heated (calcined), as in the Solvay process' final stage.
Read more :
Synthesis of Sodium Carbonate:
Solvay Process
The Solvey technique is presently the only way to make sodium carbonate. Carbon dioxide and ammonia are injected into a chilly, saturated sodium chloride solution in this process. Sodium hydrogen carbonate, which is only very little soluble in the presence of sodium ions, is virtually fully precipitated in the processes that occur. It is filtered out and burned to generate sodium carbonate. The ingredients for this procedure are affordable and widely available. Salt brine (NaCl), ammonia (NH3), and limestone (CaCO3) are the three major ingredients. CaCl2 is a significant by-product of this procedure.
The following equation can be used to represent the reactions. The hydrates are heated to create it. It can also be generated when sodium hydrogen carbonate is heated (calcined), as in the Solvay process:
2NH3 + H2O + CO2 → (NH4)2CO3
(NH4)2CO3 + H2O + CO2 → 2NH4HCO3
When common salt is added to a solution containing NH4+ and HCO3–, NaHCO3 is precipitated, which is the least soluble of the two. After that, it's filtered out.
NH4HCO3 + NaCl → NH4Cl + NaHCO3
After that, sodium bicarbonate is heated to produce Na2CO3.
2NaHCO3 → Na2CO3 + CO2 + H2O
The CO2 gas produced can be recycled.
When anhydrous sodium carbonate is dissolved in water, it recrystallizes to form washing soda crystals that include ten molecules of water during the crystallization process.
Properties of Na2CO3:
1. Physical:
Na2CO3 | Sodium carbonate |
Molecular Weight/ Molar Mass | 105.9888 g/mol |
Density | 2.54 g/cm³ |
Boiling Point | 1,600 °C |
Melting Point | 851 °C |
2. Chemical:
Heat does not affect anhydrous sodium carbonate. At 852oC, it melts without disintegration.
Hydrolysis releases OH–(aq) ions, making sodium carbonate aqueous solutions moderately alkaline.
Na2CO3(s) + 2H2O(l) → H2CO3(aq) + 2Na+(aq) + 2OH–(aq)
Sodium carbonate aqueous solutions absorb carbon dioxide from the air and create sodium hydrogen carbonate.
Na2CO3(aq) + H2O + CO2(g) → 2NaHCO3(aq)
Sodium carbonate interacts with mild vegetable acids, such as lime juice, to form carbon dioxide
Na2CO3(aq) + 2H+(aq) → 2Na+(aq) + H2O(l) + CO2(g)
Na2CO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
NCERT Chemistry Notes:
Structure of Sodium Carbonate:
Below is a diagram illustrating the structure of sodium carbonate molecules. Each molecule of sodium carbonate consists of two sodium atoms, three oxygen atoms, and one carbon atom. Each sodium cation has a positive charge, whereas the polyatomic carbonate anion has a negative charge. As a result, sodium carbonate is a neutrally charged molecule.
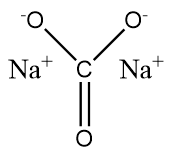
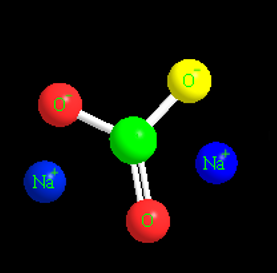
Sodium Carbonate Uses of Sodium Carbonate:
Detergents, soaps, and paper are all made with it.
Water glass (sodium silicate), borax, sodium phosphate, and a variety of other salt compounds are all made with it.
In the brick industry, it is used as a wetting agent.
It's utilised in toothpaste as an abrasive and foaming agent.
It's utilised as a pH adjuster.
It is utilised as a water softener - carbonate precipitates hard water, which contains magnesium and calcium ions.
As a standardisation reagent in the laboratory and as an analytical reagent.
Also check-