Nitric Acid - Structure, Uses, Properties, Preparation, FAQs
What is HNO3?
Nitric acid formula HNO3 is a strong acid. Spirit of Nera and Aqua Fortis are also names commonly associated with it. Pure and uncoloured, it gets a yellowish cast as it ages. Nitrogen oxides and water are formed when nitric acid decomposes. A highly toxic and corrosive substance. Severe burns are caused by it. Nitrate salts are formed when it reacts with hydroxides, metals, and oxides.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is HNO3?
- HNO3 Molecule Structure
- Sodium Nitrate Fuming
- Nitric acid - HNO3 Laboratory Preparation
- Nitric acid - HNO3 Physical Properties
- Nitric acid - HNO3 Chemical Properties
- HNO3 Structure:
- Nitric acid: Its Uses
HNO3 chemical name is Nitric acid or Chemical formula of nitric acid is HNO3. Oxidizing agents like HNO3 are used in many industrial processes. Catalytic oxidation of ammonia can be used to manufacture it. Chemicals containing this compound are often used in laboratories and are important in industrial processes such as manufacturing explosives and fertilizers. Nitric acid has a PH of roughly 3.01.
Also read -
HNO3 Molecule Structure
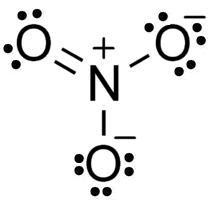
3 oxygen atoms are present in each molecule with 1 nitrogen atom and 1 hydrogen atom in each molecule. A double bond exists between both oxygen and nitrogen atoms in HNO3. In addition to being singly bonded to the nitrogen atom, another oxygen atom is also singly bonded to a hydrogen atom. Oxygen atoms with a charge of -1 are single bonded to nitrogen atoms, forming the last oxygen atom of the nitric acid molecule.
A molecule's nitrogen atom has a charge of +1 since it participates in four covalent bonds (with three oxygen atoms). So, the nitric acid molecule has a net charge of 0 (the positive charge on the nitrogen atom cancels out the negative charge on the oxygen atom). Molecular charges can be delocalized due to resonance in these molecules. Here is a diagram of the nitric acid molecule.
Sodium Nitrate Fuming
Its density is 1.50g/cm3 and it contains 98% HNO3. The explosives industry frequently uses this grade. The concentration of the anhydrous acid is approximately 21.4 M, and it is not as corrosive or volatile. The reddish-brown colour of RFNA is the result of significant amounts of dissolved nitrogen dioxide (NO2). Red fuming nitric acid has a reduced density of 1.490 g/cm3 due to the dissolved nitrogen dioxide. The addition of hydrogen fluoride (HF) can be used to make an inhibited fuming nitric acid (also referred to as an IWFNA or IRFNA). Metal tanks are coated with this fluoride for corrosion resistance. The flux protects the metal by forming a fluoride layer.

Nitric acid - HNO3 Laboratory Preparation
The principle
It is possible for a less volatile acid to displace a more volatile acid. The preparation of nitric acid in the laboratory works on this principle.
Illustration
Unlike sulphuric acid, nitric acid has greater volatility. The metal nitrates displace it with sulfuric acid.
Reactants
In a round bottom flask, 50 grams of potassium nitrate (KNO3) is combined with 25 ml of concentrated sulfuric acid (H2SO4). A temperature of about 200°C is maintained without exceeding it, as long as the reactants are not overheated.
Reaction
KNO3 + H2SO4 → KHSO4 + HNO3
(Salts of more volatile acids + fewer volatile acids displaced more volatile acids)
Collection Method
In the diagram, you can see the process of cooling and condensing the nitric acid vapours for collection.
Related Topics Link, |
Nitric acid - HNO3 Physical Properties
HNO3 | Nitric acid |
Molecular Weight/ Molar Mass | 63.01 g/mol |
Density | 1.51 g/cm³ |
Boiling Point | 83 °C |
Melting Point | -42 °C |
Nitric acid - HNO3 Chemical Properties
Acids such as nitric acid can turn blue when exposed to light.
Nitrogen dioxide is formed by the decomposition of nitric acid. As a result, it turns brown over time, despite being colourless when in its fresh form.
4HNO3 → 4NO2 + O2 + 2H2ONitric acid releases hydrogen gas with metals above hydrogen in the metal activity series.
Mg + 2HNO3 → Mg (NO3)2 + H2
Mn + 2HNO3 → Mn (NO3)2 + H2
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 7 The P-block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P-block elements
- NCERT notes Class 12 Chemistry Chapter 7 The P-block elements
HNO3 Structure:
Nitric acid: Its Uses
Producing ammonium nitrates is used to manufacture plastic, dye, and fertilizer
TNT and other explosives are made from it
Rockets with liquid fuel use it for oxidation
When it is used in its purest form, it helps remove warts
Electrochemistry uses it as a chemical doping agent
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Nitric acid is produced majorly by oxidation of ammonia via Ostwald process. Also, small amount of nitric acid are produced by treatment of sodium nitrate with sulfuric acid. Nitric acid is also component of acid rain.
A pH value below 7 and a bitter taste, along with a violent reaction to metals, are some of their properties. Nitric acid is chemically described as HNO3. White, very corrosive, colourless, and very flammable, nitric acid has an acidity of 77.
The formula of nitric acid is HNO3
H+ is removed from H2 to produce nitric acid. The conjugate acid is H3O+, hydronium; the conjugate base is NO3–, nitrate (this is the nitric acid molecule, but its H+ has been removed.
Using concentrated nitric acid on gold and platinum, however, does not oxidize them, and these metals are passivated, and hence can be dissolved with a mixture of acids or a dilute nitric solution.
NaOH neutralizes sodium bicarbonate acid. According to this, one-part nitric acid requires one-part sodium bicarbonate. Since 5 percent nitric is not very heavy, 100 gallons of the solution equals 834 pounds of nitric acid.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM