Nitrous Oxide - Laughing Gas, Structure, Preparation Applications, FAQs
What is Nitrous Oxide?
Nitrous Oxide is an oxide of nitrogen. Nitrous oxide formula is N2O.
It is also referred as DINITROGEN MONOXIDE.
It is a colourless, odourless gas having sweet smell and taste.
This inorganic compound is a linear molecule made up of 2 nitrogen and 1 oxygen atom, with nitrogen being in +1 oxidation state.
The Lewis structure of Nitrous Oxide/Dinitrogen oxide/Laughing gas is represented as follows :-
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Nitrous Oxide?
- Resonance structure of NO2
- What is Laughing gas?
- Who Discovered Laughing gas ?
- Preparation of Laughing gas :-
- Applications of Nitrous Oxide Gas OR nitrous oxide uses :-
N2O structure
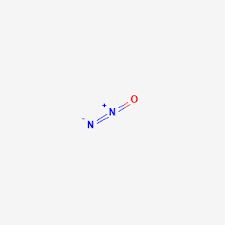
Nitrous oxide structure is a resonating structure with chemical formula N2O and having a molar weight of 44.013 g/mol.
The resonance structures of nitrous oxide can be represented as follows :-
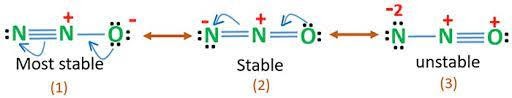
The most stable Lewis structure of nitrous oxide is ![]() where O being more electronegative possesses negative charge and the less electronegative N bears a positive charge.
where O being more electronegative possesses negative charge and the less electronegative N bears a positive charge.
Nitrous oxide is also known as laughing gas. The laughing gas formula is N2O.
Resonance structure of NO2
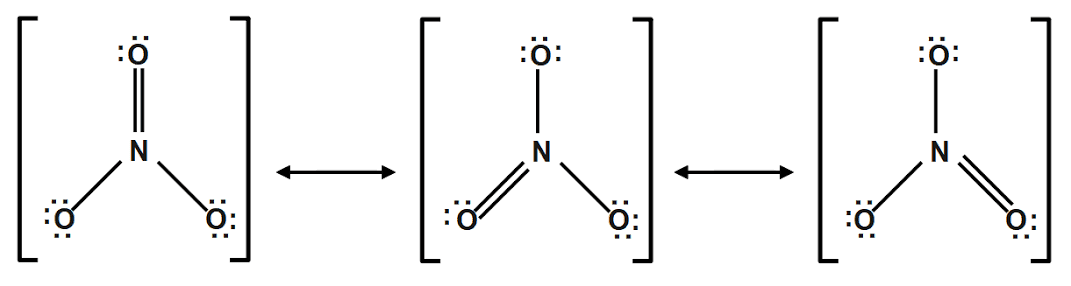
In nitrate ion one nitrogen atom and three oxygen atoms are present. In nitrate ion two types of nitrogen oxygen bonds are present. The shorter bond is stronger bond between nitrogen and oxygen.
In nitrate ion, one oxygen atom have a formal charge of 9 and other two oxygen have formal charge -1.
What is Laughing gas?
Laughing gas name is nitrous oxide. Laughing gas chemical name is nitrous oxide. Laughing gas chemical formula is N2O. It calms down the nervous system leading to slow thinking. It causes a feeling of EUPHORIA (intense feelings of pleasure) as it suppresses common sensations like physical and emotional pain, hearing and even the sensation of touch ultimately leading to slip into relaxation. Not all people laugh after inhaling this gas, some might feel sleepy also. This laughing gas is responsible for decreasing the anxiety level while undergoing a dental treatment. Nitrous Oxide is also used as a mild sedative agent in dental treatments as it helps in relieving stress and promote relaxation.
Also read :
- NCERT notes Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
Who Discovered Laughing gas ?
An English Scientist Joseph Priestley around 1772 discovered Nitrous Oxide gas or Laughing Gas was initially discovered this gas but, another English chemist – Humphry Davy, later named it and showed its psychological effect. Joseph Priestly is also famous for his work where he was successful in isolating other important gases such as oxygen and carbon dioxide. Humphrey Davy was keen to know the effects of nitrous gas or laughing gas on people and hence he started to inhale the gas and record the results.
He also took the gas outside the laboratory conditions to study its effects in detail and record his observations. He kept on increasing the quantity of gas inhaled and experienced intense impacts of the gas. He and his subjects, who were exposed to the laughing gas primarily concluded that they experienced a feeling of relaxation and calmness when exposed to the laughing gas. A highly pleasant and pleasurable sense was experienced by the people exposed to the gas, also making them laugh.
| Related topics link, |
Preparation of Laughing gas :-
Preparation of nitrous oxide involves oxidation of hydroxyl amine with sodium nitrate, coper sulphate, potassium permanganate or ferric chloride.
Reduction of nitric acid (HNO3) by stannic chloride (SnCl2) and HCl gives nitrous oxidation.
4SnCL2 + 8HCl + 2HNO3 4SnCL2 + 5H2O + N2O
Dilute HNO3 upon reacting with zinc results into formation of Laughing gas or Nitrous oxide.
Heating of ammonium nitrate results in the melting of the chemical itself eventually leading to its decomposition and exploding at 240 degrees celcius.
Heating ammonium nitrate and ammonium sulphate mixture was the first process followed in the laboratory for the laughing gas preparation.
Nitrous Oxide gas was obtained without any explosion of ammonium nitrate formed in this mixture.
(NH4)2SO4 + 2NaNO3 Na2SO4 + 2NH4NO3
The Nitrous Oxide Gas (N2O) which is obtained after heating ammonium sulphate and sodium nitrate solution is treated with KOH,FeSo4 and concentrated H2SO4.
Applications of Nitrous Oxide Gas OR nitrous oxide uses :-
Nitrous Oxide gas Is known for its anaesthetic and pain reducing
effects.This gas is not used singly but in combination with oxygen gas. 70 % nitrous oxide gas is combined with 30 % oxygen gas in anaesthetic procedures.
Nitrous Oxide gas is also a good analgesic where it is often administered as a 50% mixture with oxygen gas.
Nitrous oxide gas is more stable at room temperature and less toxic because of which it is possible to be used as an oxidizer in a rocket motor.
Initially this laughing gas was used during labour and delivery procedures.
Nowadays, it is also used in dental procedures. As it is a mild sedative agent, this gas combined with oxygen is made to inhale through a nose fitting mask.it results in dizziness and brings a sense of relaxation while undergoing the dental procedure.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
N2O chemical name is nitrous oxide.
Nitrous oxide is known as laughing gas.
Nausea , excessive shivering, fatigue are some of the ill effects caused due to the gas.
long term exposure to the gas results in memory loss causing brain and nerve damage.
Patients with cardiac problems, pregnant women (generally during first trimester) are advised not to use nitrous oxide under any circumstances. People with retinal surgeries and ear surgeries are also advised not to come in contact with nitrous oxide. Clinical professionals also avoid administration of laughing gas to patients having undergone pulmonary hypertensions, severe psychiatric problems. Effects of nitrous oxide is different on different people depending upon the amount consumed by the people. Consumption of large amount of nitrous oxide leads to loss of blood pressure, fainting and also causes a severe heart attack. Hypoxia is a condition caused due to the inhalation laughing gas and can prove dangerous if enough oxygen is not provided.
Combination of Nitrous oxide gas with other drugs or chemicals can also prove fatal for heart patients by disrupting the heartbeat. Alcohol and laughing gas when combined can lead to confusion problems, feeling of heaviness, lack of concentration and loss of body control and balance. This laughing gas is also used in the food industry for whipped cream production. It is used as a foaming reagent. Nitrous oxide is also a greenhouse gas pollutant. It is responsible for greenhouse gas pollution. This laughing gas or nitrous oxide gas emissions by humans have increased deliberately during past years. Its concentration increased up to 30% between 1980 and 2016.It has been declared as dangerous gas for existence of humans on earth.
Also Read
19 Feb'25 11:09 AM
07 Feb'25 10:38 AM
07 Feb'25 10:22 AM
07 Feb'25 10:18 AM
07 Feb'25 10:16 AM
07 Feb'25 10:11 AM
18 Oct'24 05:56 PM
18 Oct'24 10:52 AM
10 Oct'24 12:05 AM
09 Oct'24 11:41 PM