Nylon - Definition, Structure, Properties, Uses, FAQs
What is Nylon?
Nylon is a first valuable engineered material with applications fluctuating from the way of life exercises to businesses. It is a plastic that can be brought into strands or framed into step-by-step things for making comforts. You bounce across the nylon floor covering to the kitchen, have your morning meal on a nylon bowl in the wake of cleaning your teeth with a toothbrush whose fibers are made of nylon. Umbrella is made from nylon which is used during rainy days. The other applications of nylon and nylon 6 structures are discussed below.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Nylon?
- What are the properties of Nylon?
- Types of Nylon
- Two Uses of Nylon
- Nylon 6
- What is Nylon 6, 6 ?
- Nylon 6, 10
Nylon images are shown below.


Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is nylon made up of?
Nylon definition: The nylon fiber or nylon material focuses on a polymer family referenced as straight polyamides. There are two ways to deal with making nylon for fiber applications, inside the essential methodology, the particles that contain an acidic gathering (COOH) on each end respond with atoms that contain amino (NH2) bunches at each end. The nylon contains a number of carbon molecule with two amines and two acidic gathering. Henceforth, nylon 6, 6 is broadly utilized as filaments produced using nylon composition, adipic acid, and hexamethylene diamine.
The salt which is shaped by two mixtures is known as nylon and has an accurate proportion of 1:1 corrosive to base. This salt is dried and afterward warmed under a vacuum to eliminate water and structure the polymer. Nylon is prepared from petroleum.
In the other methodology, a compound that contains an amine toward one side and is corrosive at the other are polymerized to deliver a chain with rehashing units of (- NH-[CH2] n-CO-) x. The nylon fiber is alluded to as nylon 6 if n = 5 which is one more typical type of this polymer. The business creation of nylon 6 begins with caprolactam that utilizes an open-ring polymerization.
In both the methodologies, the polyamide chemical structure is softened and attracted in the wake of cooling to acquire the ideal properties of every intended use.
Things made of nylon are rope, sleeping bags, tarpaulin, etc.
What are the properties of Nylon?
Different characteristics of nylon are shown below.
Glistening
Flexible
Extremely impressive
Harm impervious to oil and numerous synthetic compounds
Strong
Doesn't absorb water
Dries rapidly
Types of Nylon
Nylon 6 – it had been created by Paul Schlack. It's shaped by ring-opening polymerization.
Nylon 510 – it's acquired from sebacic and Penta methylene diamine corrosives.
Nylon 1,6 – it's delivered from nitriles with the help of corrosive catalysis.
Nylon 6, 6 – Wallace Carothers protected nylon 6, 6 with the utilization of amide.
Two Uses of Nylon
Nylon applications are-
Clothing – Shirts, Foundation pieces of clothing, parkas, clothing, swimwear, and cycle wear.
Industrial utilizes – Conveyer and safety belts, parachutes, nets and ropes, canvases, string.
Other Nylon uses,
It's utilized to make a fishnet.
It's used as plastic in manufacturing machine parts
Further, we will discuss Nylon 6, Nylon 6 properties and uses.
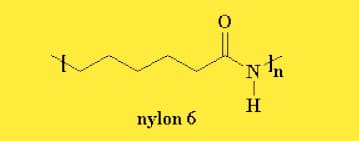
Nylon 6
Nylon 6 or polycaprolactam is a polymer made by Paul Schlack at IG Farben to copy the properties of nylon 6,6 without dismissing the patent on its creation. (Around a similar time, Kohei Hoshino at Toray additionally prevailed with regards to integrating nylon 6.) It is a semicrystalline polyamide. In contrast to most different nylons, nylon 6 isn't a build-up polymer, yet rather is framed by ring-opening polymerization; this makes it a unique case in the correlation among build-up and expansion polymers.
Its opposition with nylon 6,6 and the model it set has additionally formed the financial matters of the engineered fiber industry. Nylon monomer is hexamethylenediamine and adipic acid. Nylon 6 has the center attributes of any individual from the nylon family, including its hardness, solidness, and durability. It has great mechanical hosing and protection characteristics.
| Related topics link, |
Nylon structure is shown below.
It slides with the insignificant scraped area, opposes weariness, and is very wear-safe over the long run. As an additional advantage, it has great machinability and can be worked into its last structure decently without any problem. It likewise has a glossy completion.
Properties of Nylon 6
Nylon 6 strands are extreme, having high rigidity, versatility, and shine. They are wrinkle-proof and profoundly impervious to the scraped areas and synthetic substances like acids and salts. The filaments can assimilate up to 2.4% of water, albeit this brings down rigidity. As an engineered fiber, Nylon 6 is, for the most part, white yet can be colored in an answer shower before creation for various shading results. Its determination is 6–8.5 gf/D with a thickness of 1.14 g/cm3. Its dissolving point is at 215 °C and can secure warmth up to 150 °C on average.
Uses of Nylon 6
The application of Nylon 6 is in modern yarn. It is found in ropes, hardcore texture, toothbrush strands, and numerous different items that depend on extreme modern nylon for a mix of solidarity and adaptability. Nylon 6 is likewise found in textures with a specific kind of positive design sheens like hosiery, chiffon, and organza. This is because of the glossy surface completion that is extraordinary to Nylon 6.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 15 Polymers
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 15 Polymers
- NCERT notes Class 12 Chemistry Chapter 15 Polymers
What is Nylon 6, 6 ?
The monomer of Nylon 6, 6 are hexamethylenediamine and adipic acid. Nylon 6, 6 polymer resembles Nylon 6 yet not the same. At 160 degrees Celsius, Nylon 6.6's sparkle age strength at break is 13.6 kg versus Nylon 6's 12.0 kg.
Precisely when you increment the temperature to 180℃, the capability becomes starker. Nylon 6.6's sparkle age strength is 11.5 kg, while Nylon 6's drops down to 2.6 kg. In explicit applications, this could have a tremendous effect.
Some cutting-edge fiber measures, like tire rope, use warmth to combine the fiber into the end-product. Nylon 6, 6 polymers withstand these cycles and continue to give reliable strength well from that point. Nylon 6, alternately, ruins or powers a change of the cycles, decreasing handiness.
Nylon 6, 6 polymers offer low grouch, incredible stretch recovery, and higher scratched region resistance than Nylon 6 and most various materials you'll find in the business place. In all honesty, its scratched spot and warmth block are the describing properties that make it an unparalleled choice for present day applications.
The fibers in Nylon 6, 6 are 33% more scratched spot protected than Nylon 6 strands, withstanding in excess of 60,000 cycles over Nylon 6's 40,000 cycles. In hard-wearing mechanical fiber applications, this infers significantly stronger long stretch execution.
Nylon 6, 6 chemical structure.
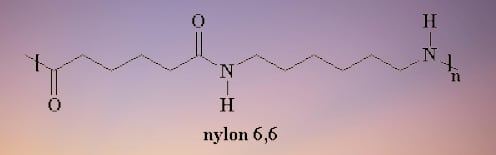
Uses of nylon 6,6
Nylon 6.6 has a couple of fundamental uses, including smaller than expected denier versus significant denier versus inventive. In its small denier structure, it is generally used in clothing. It offers amazing solidness and wears resistance for prevalent sports gear and current workwear. In its significant denier structure, it is used in tires and present-day things. In its creative construction, you'll see it in auto airbags, parachutes, cover, and various uses where preposterous execution, strength, and resolute quality are totally basic.
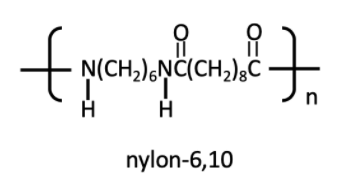
Nylon 6, 10
Nylon 6, 10 is a polymer prepared from hexamethylene diamine and sebacic acid. Nylon 6, 10 has a lower melting point. It has higher resilience. Nylon 6, 10 is used in toothbrushes, paintbrushes.
Nylon 6,10 structure
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Uses of nylon-
It's utilized to make a fishnet.
It's used as plastic in manufacturing machine parts
The monomers of nylon 66 are adipic acid and hexamethylene diamine.
Suitable monomers are joined to shape a long chain through a course of the build-up polymerization response.
Nylon is a very strong fiber and it is flexible. Therefore, used for making bristles of toothbrush.
Nylon 6 is formed from one monomer. The monomer of nylon 6 contains six carbon atoms which depict the name nylon 6. Nylon 6, 6 is formed from two monomers which contain total of 12 carbon atoms.
Also Read
21 Nov'24 06:25 PM
20 Oct'24 10:51 AM
19 Oct'24 10:26 AM
19 Oct'24 10:23 AM
19 Oct'24 10:16 AM
30 Sep'24 12:20 PM
30 Sep'24 11:36 AM
Articles
Questions related to
Correct Answer: Nylon 66
Solution : The correct option is Nylon 66.
The polymer formed by the reaction of hexamethylene diamine with adipic acid is known as "Nylon 66". It is a synthetic polyamide made up of repeating units produced from these two monomers. Nylon 66 is well-known for its strength, durability, and wide range of uses, which include textiles, industrial products, and engineering components. The reaction can be represented as follows: Hexamethylene diamine + Adipic acid → Nylon 66 + Water.