Orthoboric Acid
Orthoboric acid, H₃BO₃, is a weak acid derived from boron—an element naturally available in nature. It usually occurs as a white, crystalline solid or as a powder and easily dissolves in water. This compound has antiseptic, antifungal, and insecticidal properties and is added to various medicines and household products. This further contains one boron atom, three oxygen atoms, and three hydrogen atoms—a triangular planar structure promoted chemically. Even though this is a simple compound, the chemical behavior of orthoboric acid can be considered quite interesting. Orthoboric acid acts as a Lewis acid by accepting electron pairs.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Forms and Types of Orthoboric Acids
- Applications and Relevance
- Some Solved Examples
- Summary
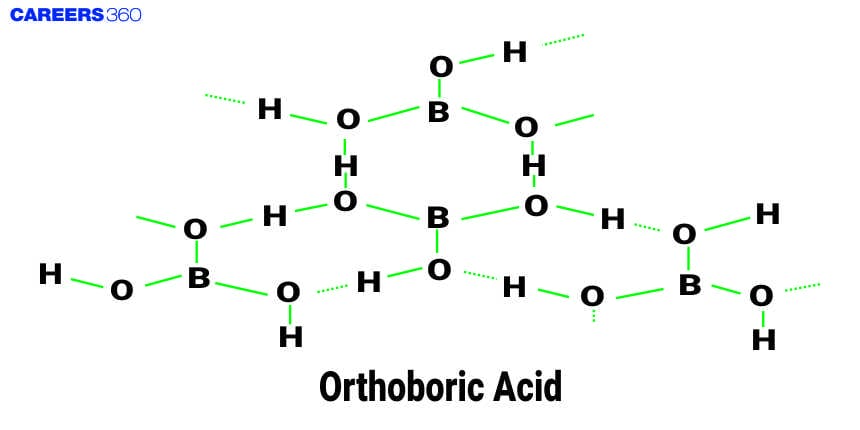
Orthoboric acid, H3BO3 is a white crystalline solid, with soapy touch. It is sparingly soluble in water but highly soluble in hot water. It can be prepared by acidifying an aqueous solution of borax.
Na2 B4O7+2HCl+5H2O→2NaCl+ 4B(OH)3
It is also formed by the hydrolysis (reaction with water or dilute acid) of most boron compounds (halides, hydrides, etc.). It has a layer structure in which planar BO3 units are joined by hydrogen bonds as shown in the figure given below.
Boric acid is a weak monobasic acid. It is not a protonic acid but acts as a Lewis acid by accepting electrons from a hydroxyl ion:
BOH3+2HOH→[B(OH)]−+H3O+
On heating, orthoboric acid above 370K forms metaboric acid, HBO2 which on further heating yields boric oxide, B2O3
H3BO3→ΔHBO2→ΔB2O3

Forms and Types of Orthoboric Acids
The different types or forms of orthoboric acids have characteristic typical features and applications. The hydrated orthoboric acid type is the most commonly used in domestic and pharmaceutical applications. Because of the high reactivity of boric acid in anhydrous conditions, it finds applications in several industrial processes, including the manufacture of glass and metallurgy applications. The fact that boric acid could exist in complex compounds as borates with other elements led to their formation—these have applications in ceramics, and detergents as well as preservatives. These forms, thus underpin the complex behavior of orthoboric acid and its applications, which cut across a wide spectrum.
Applications and Relevance
Orthoboric acid finds very broad and significant practical applications. In everyday life, it is used in antiseptics and eyewashes, and it is also a mild pesticide. It is also effective against fungal diseases, as it is greatly effective against such problems as athlete's foot and yeast infections. For industrial purposes, the strength and heat resistance of orthoboric acid in specialized glass products increase quality, such as fiberglass and ceramics, are greatly improved by its use in their production. It finds an application in chemistry as a forerunner for the synthesis of other boron compounds and as a catalyst for some organic reactions.
Recommended topic video on (Orthoboric Acid)
Some Solved Examples
Example 1
Question: Boric acid is polymeric due to:
- Its acidic nature
- The presence of hydrogen bond
- Its monobasic nature
- Its geometry
Solution: As we have learned, boric acid has a layer structure in which planar H₃BO₃ units are linked by hydrogen bonds. Therefore, the correct answer is option (2).

Example 2
Question: When orthoboric acid is heated to red heat, the residue is:
- Boron
- Boric oxide
- Metaboric acid
- Polyboric acid
Solution: Orthoboric acid, H₃BO₃, when heated above 370K, forms metaboric acid (HBO₂). On further heating, it yields boric oxide (B₂O₃). Therefore, the correct answer is option (2).
Example 3
Question: Orthoboric acid is used as:
- It is used in making optical and hard glass
- It is used as an antiseptic
- It is used as a food preservative
- All of the above
Solution: Orthoboric acid, H₃BO₃, is used in making optical and hard glass, as an antiseptic, and as a food preservative. Therefore, the correct answer is option (4).
Summary
Orthoboric acid is a versatile compound that finds wide applications both in everyday life and specialized industries. From the simplest and most fundamental properties and forms to practical and academic relevance, orthoboric acid presents a multifaceted compound and is important. It has a stringent place among medical treatments, household products, and industrial processes—the epitome of utility and adaptability. The characteristics and applications of orthoboric acid highlight information on the integral place this compound holds within modern science and technology.
Frequently Asked Questions (FAQs)
Orthoboric acid is also boric acid and holds the chemical name H₃BO₃. It is a weak acid derived from boron. At room temperature, it appears like a more or less white odorless crystalline solid or powder. It bears antiseptic, antifungal, and insecticidal properties.
It is contained in a number of household materials: antiseptics, eyewash solutions, and insecticides. It contains antifungal and antimicrobial activities; hence, it finds the same applications in minor cautery for wounds and eye irritation and killing unwanted pests like ants and cockroaches.
This chemical is quite important industrially in the manufacture of fiberglass, ceramics, and glass products as it improves their strength and promotes resistance to thermal shock. This chemical is further used as a precursor for other boron compounds and as a catalyst in organic chemical reactions.
Although generally safe, this acid can be dangerous upon ingestion or handling and therefore should be kept out of children's and pets' reach. As such, the precautions against this element need to be carried out at all times when dealing with it.
One of the very unique properties of orthoboric acid is its ability to act like a Lewis acid by forming complex compounds. Thus, it has a significant role in chemical research for developing new materials or studying reaction mechanisms and seeking some technological applications.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM