Oxidizing Agent - Definition, Examples, Applications, Uses, FAQs
An oxidizing agent is a chemical that takes electrons from other reactors during a redox reaction. The oxidizing agent usually takes those electrons for itself, acquiring electrons and reducing them. As a result, an oxidizing agent is an electron acceptor. A species capable of transferring electronegative atoms (particularly oxygen) to a substrate is also known as an oxidizing agent.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Redox Reaction:
- What is an Oxidizing Agent?
- Oxidizing Agent Examples of Oxidizing Agents:
- Applications of Oxidizing Agent:
Redox Reaction:
Redox reactions are oxidation-reduction chemical reactions in which the oxidation states of the reactants change. The term'redox' refers to the reduction-oxidation process. All redox reactions can be divided down into two types of reactions: reduction and oxidation.
In a redox reaction, or Oxidation-Reduction process, the oxidation and reduction reactions always happen at the same time. The oxidizing agent is the substance that is being reduced in a chemical process, while the reducing agent is the substance that is being oxidised.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is an Oxidizing Agent?
An oxidizing agent (also known as an oxidizer or oxidant) is a chemical species that tends to oxidise other substances, causing them to lose electrons and hence raise their oxidation state. Halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide (H2O2) are all oxidising power examples of oxidizing agents.
Definition for Oxidizing Agent
Oxidizing agents can be defines in either of the 2 ways:
As an electron acceptor: they are chemical compounds whose atoms in a chemical reaction remove at least one electron from another atom. Oxidizing agents are reactants that undergo reduction in redox reactions, according to this definition.
As an atom-transfer substance: In a chemical process, an oxidizing agent is a substance that transfers at least one electronegative atom to a chemical species. Typically, the transferred atom is an oxygen atom. The transfer of an electronegative atom between two reactants is involved in a number of combustion processes and organic redox reactions.
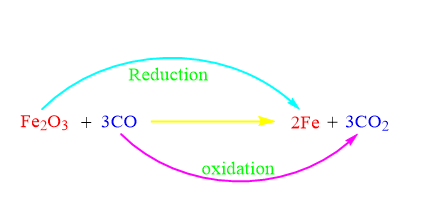
The Fe2O3 molecule serves as an oxidant in the oxidising agent example above by transferring an electronegative oxygen atom to the carbon monoxide molecule.
The dangerous goods definition of an oxidizing agent:
An oxidizing agent, according to the dangerous goods definition, is a substance that can cause or contribute to the combustion of another substance. As a result, some materials classified as oxidizer by analytical chemists are not classified as oxidizing agents in the dangerous goods sense. One oxidizing agent example is potassium dichromate, which fails the oxidizin's dangerous goods test.
Factors Affecting Oxidizing Power of Oxidizing Agent:
Oxidizing agents are usually found in their most oxidised states, which means they have a significant ability to gain electrons and undergo reduction. Ions, atoms, and molecules with a high affinity for electrons are thought to be good oxidizers. The greater the oxidizing power, the stronger the electron affinity.
Fluorine is thought to be the most powerful elemental oxidizer. This could be because fluorine is the most electronegative element in the present periodic table, and hence has the highest attractive force on electrons of all the elements. In fact, diatomic fluorine (F2) has such a powerful oxidizing activity that it can cause metals like asbestos and quartz (as well as molecules like water) to explode into flames when exposed to it.
Diatomic oxygen (O2), diatomic chlorine (Cl2), and ozone (O3) are some more oxidizing agent examples of elemental oxidizing agents.
In a redox process, the standard electrode potential of a half-reaction gives information on the chemical substance's oxidizing power. Below is a diagram that ranks some oxidizers according to their oxidizing capability.
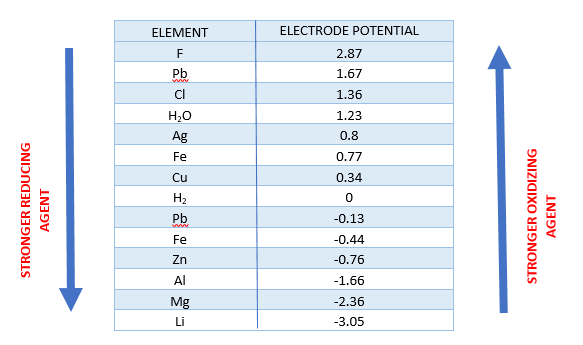
Some chemicals with substantial oxidation states can also be used as oxidizers. The permanganate ion, chromate ion, and dichromate ion are all ionic oxidizing agent examples. Nitric acid, perchloric acid, and sulphuric acid are acidic oxidizing agent examples of excellent oxidizers. The electronegativity of molecules increases as the oxidation states of the atoms increase, increasing their absorption.
Related Topics Link, |
Oxidizing Agent Examples of Oxidizing Agents:
Halogens:
Halogens are the 17th group elements of the periodic table that make up the group. They are regarded to have a significant ability to gain electrons, owing to their high electronegativities in comparison to other groupings of elements. This indicates that they have the ability to easily attract electrons to their nuclei. Iodine, bromine, chlorine, and fluorine are oxidizing agent examples of halogens that are good oxidizing agents. Because of its high electronegativity, fluorine is regarded to be the most powerful elemental oxidizing agent.
Oxygen:
Oxygen is the element with the atomic number of 8 and is represented by the letter O. It is a highly reactive non-metal with good oxidizing capabilities that belongs to the chalcogen group of the periodic table. Metals, in general, tend to create metal oxides when they react with ambient oxygen, owing to oxygen's high oxidizing power. The bulk of combustibles are found to contain oxygen.
Hydrogen perioxide:
The chemical compound hydrogen peroxide has the formula H2O2. It appears to the naked eye as a colourless liquid with a viscosity greater than water. The simplest chemical with a peroxide functional group and an oxygen-oxygen single bond is hydrogen peroxide. It's used as a disinfectant, bleaching agent, and a weak oxidizing agent.
Ozone:
Ozone is a powerful oxidizing agent because a molecule of nascent oxygen that is more reactive than oxygen decomposes fast to give. Strong oxidizing agents absorb and reduce electrons, and are typically defined by halogens or an oxygen-containing product. It will readily hand it on to another material. These compounds' oxidation status is deteriorating.
When ozone decomposes, it releases nascent oxygen.
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 8 Redox Reaction
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 8 Redox Reaction
- NCERT notes Class 11 Chemistry Chapter 8 Redox Reaction
Applications of Oxidizing Agent:
Oxidizing agents have a wide range of commercial and industrial uses. The following are a handful of these uses.
Fabrics are bleached using oxidizing agents .
Water purification.
An oxidizing agent is used in the combustion of fuel.
Batteries that are used to store energy make use of oxidizers.
Rubber vulcanization for increasing the strength and the elasticity of rubber is carried out by an oxidizer.
Many biological functions, such as metabolism and photosynthesis, require oxidizing agents.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
An oxidizing agent (also known as an oxidizer or oxidant) is a chemical species that tends to oxidise other substances, causing them to lose electrons and hence raise their oxidation state
Redox reactions are oxidation-reduction chemical reactions in which the oxidation states of the reactants change. The term'redox' refers to the reduction-oxidation process. All redox reactions can be divided down into two types of reactions: reduction and oxidation.
Give an application of oxidizer
Answer: An oxidizing agent is used in the combustion of fuel.
Batteries that are used to store energy make use of oxidizers.
Rubber vulcanization for increasing the strength and the elasticity of rubber is carried out by an oxidizer
Fluorine is thought to be the most powerful elemental oxidizer. This could be because fluorine is the most electronegative element in the present periodic table, and hence has the highest attractive force on electrons of all the elements
The most common oxidizing agents are Oxygen, Hydrogen Peroxide, Ozone, etc.
Also Read
04 Jul'22 05:40 PM
04 Jul'22 05:35 PM
04 Jul'22 05:33 PM
04 Jul'22 05:30 PM
04 Jul'22 05:28 PM