Redox Titration - Definition, Examples, Types, Principle, FAQs
Have you ever wondered how chemists precisely determine the amount of a substance that can gain or lose electrons in a reaction? How do we measure the exact quantity of an oxidizing or reducing agent in a solution? Answer to all these question is Redox titration. Redox Titration is a analytical method to determine the concentration of a provided analyte by triggering a redox reaction between the titrant and provided analyte of unknown strength. These types of titrations sometimes require the use of a potentiometer or a redox indicator.
This Story also Contains
- What is Redox Titration
- Reduction
- Oxidation
- Redox Titration Principle
- Indicators
- Redox Titration Example
- Types Of Redox Titration
- Some Solved Examples
What is Redox Titration
A Redox titration is a type of titration in chemistry that involves a reduction–oxidation (redox) reaction between the analyte (the substance being analyzed) and the titrant (the solution of known concentration). Unlike acid–base titrations, which rely on neutralization, redox titrations rely on the transfer of electrons.
Redox titration is based on an oxidation-reduction reaction between the titrant and the analyte. It is one of the most common laboratory methods to identify the concentration of unknown analytes.
In order to evaluate redox titrations, the shape of the corresponding titration curve must be obtained. In these types of titration, it proves convenient to monitor the reaction potential instead of monitoring the concentration of a reacting species.
As discussed earlier, redox reactions involve both oxidation and reduction. The key features of reduction and oxidation are discussed below.
Reduction
A substance can undergo reduction can occur via:
- The addition of hydrogen.
- The removal of oxygen.
- The acceptance of electrons.
- A reduction in the overall oxidation state.
Oxidation
The following points describe a substance that has undergone oxidation.
- The addition of oxygen.
- Removal of hydrogen which was attached to the species.
- The donation/loss of electrons.
- An increase in the oxidation state exhibited by the substance.
Thus, it can be understood that redox titrations involve a transfer of electrons between the given analyte and the titrant.
Example of redox titration: Reacting iodine solution with any reducing agent. In this titration starch is used as an indicator.
Redox Titration Principle
The redox reaction is defined as the reaction between oxidizing agent and reducing agent. The redox reaction is also known as oxidation-reduction reaction. During the chemical reaction, the electrons is transferred to the reacting ions of the aqueous solution.
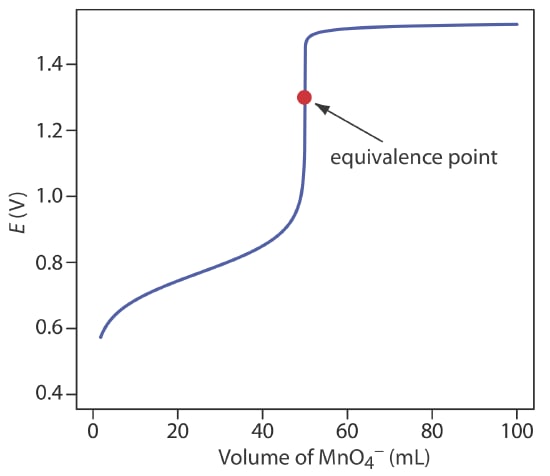
Redox titration curve
To determine the redox titration value, the shape of the titration curve is evaluated. The titration curve helps is determine the hydronium ion concentration (H3O+ ) when the titrant is added.
Indicators
The redox indicator undergoes a colour change at specific electrode potential when added to the solution.
To undergo fast and reversible colour change, the equilibrium in the redox titration needs to be established quickly.
Common Indicators of Redox Indicators
- Phenanthroline metal complex and bipyridine metal complex. Here, the metal changes oxidation state.
- Methylene blue.
- The organic compounds are the most common redox indicator.
- 2,2'- Bipyridine
Related Topics Link,
Redox Indicators Examples
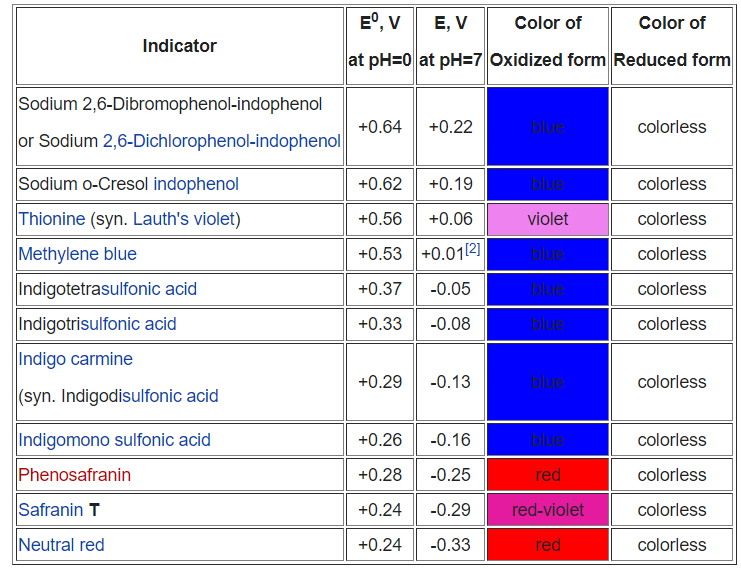
pH Dependent
pH Independent

Redox reaction as the basis for titration
In redox titrations, oxidation-reduction reaction take place. In this reaction redox sensitive indicators are used to determine the strength of oxidant or reductant.
In this reaction oxidising agents like potassium permanganate, potassium dichromate and iodine are used.
Determining The Strength Of KMnO4 Using Standard Oxalic Acid Solution
In the titration, the analyte used is oxalic acid and the titrant used is potassium permanganate. The oxalic acid acts as a reducing agent, and the KMnO4 acts as an oxidizing agent. Since the reaction takes place in an acidic medium, the oxidizing power of the permanganate ion is increased. This acidic medium is created by the addition of dilute sulfuric acid.
$\mathrm{MnO}^{-4}+8 \mathrm{H}^{+}+5 \mathrm{e}^{-} \rightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_2 \mathrm{O}$
Molecular Equation
$2 \mathrm{KMnO}_4+3 \mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{~K}_2 \mathrm{SO}_4+2 \mathrm{MnSO}_4+3 \mathrm{H}_2 \mathrm{O}+5[\mathrm{O}]$
$\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \cdot 2 \mathrm{H}_2 \mathrm{O}+[\mathrm{O}] \rightarrow 2 \mathrm{CO}_2+3\left[\mathrm{H}_2 \mathrm{O}\right] \times 5$
Complete Reaction
$2 \mathrm{KMnO}_4+3 \mathrm{H}_2 \mathrm{SO}_4+5 \mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \cdot 2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{K}_2 \mathrm{SO}_4+2 \mathrm{MnSO}_4+18 \mathrm{H}_2 \mathrm{O}+10 \mathrm{CO}_2$
KMnO4 is used as an indicator where the permanganate ions are a deep purple colour. In this redox titration, MnO4– is reduced to colourless manganous ions (Mn2+) in the acidic medium. The last drop of permanganate gives a light pink colour which shows that the endpoint is reached. The following chemical equation can represent the reaction that occurs.
Potassium permanganate is a dark purple coloured liquids which is a strong oxidising agent. In these titration indicators are not utilised as potassium permanganate acts as a self indicator.

During the titration, the pink colour of potassium permanganate is discharged till the reductant is oxidised. As the solution gives tinge pink colour with the addition of single drp of potassium permanganate, the end point of titration is indicated.

The redox reaction where the oxidising agent does not act as the self-indicator, diphenym amine or N-phenylanthranilic acid is used.
In iodimetric titrations, iodine is directly titrated against a reducing agent.
Ex: In determining the hypo (or) sodium thiosulphate, iodine directly reacts with sodium thiosulphate. Here, starch is used as an indicator and produces an intense blue colour.
In Iodimetric titration, addition of an oxidising agent to excess iodide ions to produce iodine takes place which is then titrated with standard thiosulphate solution.
Also Read:
Redox Titration Example
An example of a redox titration is the titration of potassium permanganate (KMnO4) against oxalic acid (C2H2O4). The procedure and details of this titration are discussed below.
Titration of Potassium Permanganate against Oxalic Acid
- Prepare a standard Oxalic acid solution of about 250 ml.
- The molecular mass of oxalic acid is calculated by adding the atomic mass of each constituent atom
- The $\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \cdot 2 \mathrm{H}_2 \mathrm{O}$ molecular mass is 126
- The weight of oxalic acid needed to prepare 1000 ml of 1M solution is 126 g. Hence, the weight of oxalic acid needed to prepare 250 ml of 0.1 M solution is
$126 / 1000 \times 250 \times 0.1=3.15 \mathrm{~g}$
Types Of Redox Titration
- In Bromometry, bromine (Br2) titrant is used.
- In Cerimetry, cerium (IV) salts is used.
- In Dichrometry potassium dichromate is used.
- In Iodometry, iodine (I2) is used.
- Permanganometric titration uses potassium permanganate.
Application Of Redox Titration
- Redox titration is applied to analyse inorganic analytes. A redox titration is helpful in determining the concentration of unknown analyte using a standard titrant.
- It is also utilised to analysis of organic analytes.
- It is used in the industrial production of cleaning products. Nitric acid is formed from the oxidation reaction of ammonia. Redox reactions are also applied in the process of electroplating.
Also check-
Some Solved Examples
Question.1 Which of the following acts as a self-indicator in redox titrations?
a) Potassium dichromate
b) Potassium permanganate
c) Iodine
d) Ferrous sulphate
Solution:
KMnO₄ is purple and turns colorless after reduction, so no external indicator is required.
Hence, the correct answer is option (b) Potassium permanganate
Question.2 In the titration of oxalic acid with KMnO₄ in acidic medium, oxalic acid is:
a) Oxidized to carbon dioxide
b) Reduced to formic acid
c) Oxidized to carbon monoxide
d) Reduced to methane
Solution:
Oxalic acid $\left(\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4\right)$ is oxidized to $\mathrm{CO}_2$ while $\mathrm{MnO}_4^{-}$ is reduced to $\mathrm{Mn}^{2+}$.
Hence, the correct answer is option (a) Oxidized to carbon dioxide
Question.3 In acidic medium, the equivalent weight of $\mathrm{KMnO}_4$ is:
a) M/2
b) M/3
c) M/5
d) M/1
Solution:
In acidic medium,$\mathrm{MnO}_4^{-}$(oxidation state +7 ) is reduced to $\mathrm{Mn}^{2+}(+2)$, i.e., 5 electrons transferred $\rightarrow$ Eq. wt. $=\mathrm{M} / 5$.
Hence, the correct answer is option (c) M/5
Practice More Question With Link Given Below
| Types of Redox Reactions practice question and MCQs |
| Balancing of Redox Reaction: Ion Electrode Method practice question and MCQs |
Frequently Asked Questions (FAQs)
The common reducing agents that are used in redox titrations are - Fe²⁺, S₂O₃²⁻, MnO₄⁻, Vitamin C or ascorbic acid, Benedict's reagents to reduce cupric ions to form cuprous ions, manganese (II) sulfate to reduce oxygen in the water, etc
Titrant is a substance (such as a reagent solution of precisely known concentration) that is added in titration.
Phenanthroline , starch
It is used to determine the concentration of unknown analyte.
Bromometry, iodometry
