Partial Pressure - Dalton’s Law, Application, Formula, Units, FAQs
After reading this article, the reader should be able to answer the following questions:- how to calculate partial pressure, how to find partial pressure experimentally, what is partial pressure.
Keywords - partial pressure, partial pressure units, partial pressure meaning, partial pressure formula, partial pressure of the gas.
Partial pressure is the pressure exerted by an individual gas present in a mixture of gases provided that the gases should be non-reactive.
The partial pressure of an individual gas is determined by a formula
pn=Xnptotal
Partial Pressure-
The physical state of the gaseous state can be described by quantities like volume, pressure, and temperature. The origin of the term “Atmospheric Pressure” is from the fact that the pressure we are talking about is exerted by Earth’s atmosphere. The gravity of Earth pulls air toward its surface which develops pressure on the earth’s surface. This is how pressure develops on the Earth’s surface. One of the main characteristics of air is that it exerts pressure in all directions in the same quantity. The First Gas law given by Boyle, established an inverse relationship between pressure and volume and Gay Lussac’s Law gave a direct relationship between pressure and temperature.
Partial Pressure definition- Partial Pressure is defined as the pressure exerted by an individual gas in a mixture of non-reactive gases.
Partial pressure meaning is that pressure which is not total but only the pressure of an individual gas is taken out of the total pressure of mixed gases. This pressure is called partial pressure.
The partial balancing means balancing partially reciprocating masses.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Partial Pressure-
- Dalton’s law of partial pressure applicability-
- Partial pressure formula-
- Units of Partial Pressure-
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Dalton’s Law of Partial Pressure-
Dalton was the first scientist to state that the pressure of gas doesn’t depend on the nature of the gas. Just how pure gases obey gas laws, a mixture of gases also obey gas laws i.e., gas laws apply to oxygen as well as air.
Each non-reacting gas present in the air contributes to the total pressure in proportion to a fraction (in moles). This contribution of each gas is known as the partial pressure of the gas.
Dalton stated that the total pressure of a non-reactive gaseous mixture will be equal to the sum of partial pressure of each gas.
Ptotal is the total pressure of the gases and p1, p2, p3 .. are the partial pressure of each gas. Partial pressure is the same pressure exerted by an individual gas if the gas is contained in a jar of the same volume and temperature.
Dalton’s law of partial pressure is not applicable to the mixture of gases that reacts with each other.
Application of Dalton’s Law of Partial pressure of gas-
The pressure of gases over the surface of the liquid can be calculated by using Dalton’s Law of partial pressure. The total pressure of a mixture of gas and water will be equal to atmospheric pressure if the water level inside and outside the vessel are equal.
The gas present over water exerts combined pressure due to its vapor pressure and due to the pull of gravity. This gas contains water vapors. The pressure of dry gas can be calculated easily using the following equation.
When the level of water gets equal on the inside and outside-
Patm=Ptotal
Ptotal=Pgas+Pwater
Pgas=Patm-Pwater
Pwater=Aqueous tension
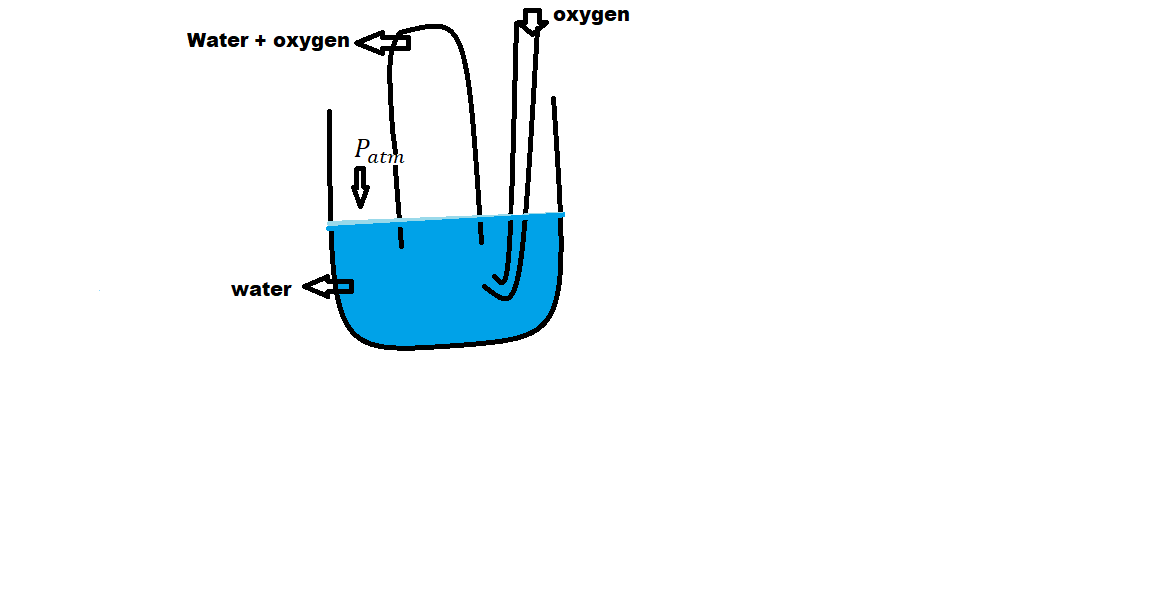
Fig- Pressure measurement of gas over the surface of the water.
The vapor pressure of a gas is different at a different temperature, therefore, Pwater is known.
Aqueous tension is the pressure exerted by water vapors. This Aqueous tension is the vapor pressure. Vapor pressure is the pressure exerted by the vapors of the liquid. Above the surface of a liquid, there always exists vapors of that liquid which exist in equilibrium with the water.
Dalton’s law of partial pressure applicability-
Dalton set a restriction for gases obeying Dalton’s law of partial pressure.
Gases of the mixture should not react with each other. Some gases like ammonia (NH3 and HCl) react with each other to form products like ammonium chloride (NH3Cl). Therefore, Dalton's law of partial pressure does not apply to such gases.
Similarly, gases like Hydrogen(H2) and chlorine (Cl2) react to form hydrogen chloride (HCl).
Related Topics Link, |
Partial pressure formula-
Let there be a container with gas A and gas B inside it. Gas A and Gas B will have partial pressure pa and pb.
According to the ideal gas equation-
pa=n1RT/V
And pb=n2RT/V
Here, n1 and n2 are numbers of moles of gases present in the container.
the total pressure of a mixture of two gases is given by-
ptotal=p1+p2
=n1 RT/V+n2 RT/V
=(n1+n2) RT/V
Dividing p1 by ptotal-
p1/ptotal=(n1/n1+n2) RTV/RTV
n1/n1+n2= n1/n2= X1
Where X1 is the mole fraction of the first Gas.
p1=X1 ptotal
For both gases A and B,
The general equation can be written as-
pn=Xn ptotal
This is the formula of partial pressure of any gas.
Where pn is the partial pressure of nth gas and xn is the partial pressure of nth gas.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 2 Solutions
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 2 Solutions
- NCERT notes Class 12 Chemistry Chapter 2 Solutions
Units of Partial Pressure-
Partial pressure units are the same as the units of normal pressure.
The barometer is the instrument used in the lab to measure the pressure. The height of mercury determines the atmospheric units.
The barometer is assembled using a glass tube. This glass tube is closed from one end and open from the other end. Glass tube is filled with pure mercury. Glass tube is inverted into an open vessel containing mercury. The level of mercury drops until the atmospheric pressure acting outside the glass tube becomes equal to the pressure inside the tube.
If the height of the column decreases then atmospheric pressure increases and if the height of the column increases then the atmospheric pressure decreases.
It is the maximum height of mercury that determines the pressure. The height keeps falling and raising due to the changes from time to time.
Consider mercury-filled in an inverted glass tube of h cm and area of cross-section be equal to a cm2
We know that
pressure=force/area
Force=mass×acceleration
pressure=mass×acceleration/area
P=mg/A
P=d×V×g/A
P=d×A×h×g/A
=hdg
where d is the density, h is the height, g is the gravity and A is the area.
1 atm is that pressure which supports a mercury height of 76 cm at 0°C
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Partial pressure is the pressure exerted by an individual gas present in a gaseous mixture provided that all the gases should be non-reactive. Unlike volume, the pressure of a gas is independent of the nature of the gas under consideration. Therefore, Dalton gave a relationship between the total pressure of a gaseous mixture and the partial pressure of an individual gas.
From the ideal gas equation, the relationship between the total pressure of the gaseous mixture, partial pressure of the individual gas, and its mole fraction can be derived.
The general formula of partial pressure is given by
pn=Xn ptotal
To find the partial pressure of gas first find out the total pressure of the mixture then calculate the mole fraction of the same gas. Mole fraction is the mass of that particular gas divided by the mass of the total mixture.
Following is the relation between partial pressure and total pressure of the gas.
pn=Xn ptotal
Where pn is the partial pressure of the nth gas, xn is the mole fraction of that gas and total is the total pressure of the gaseous mixture.
Oxygen has a partial pressure of 160 mm of Hg. The standard value is where 1 atm of pressure is supported by 760 mm of Hg at sea level. 760 mm of Hg is the height of mercury in a glass tube which supports a height of 760 mm of Hg. Depending on the changing time and place height of mercury keeps changing.
The partial pressure of helium is 203 mm of Hg and oxygen has a partial pressure of 160 mm of Hg.
Also Read
19 Feb'25 06:42 PM
19 Feb'25 06:38 PM
19 Feb'25 06:35 PM
19 Feb'25 06:29 PM
19 Feb'25 06:23 PM
19 Feb'25 05:11 PM
25 Oct'24 05:23 PM
18 Oct'24 10:46 AM
10 Oct'24 12:09 PM
10 Oct'24 12:06 PM