PCl5 Hybridization - Lewis Structure, Structure, Geometry FAQs
Have you ever thought about how a single atom can form bonds of equal strength and shape, even when its orbitals are different? The answer is hybridization. Hybridization is defined as the formation of a new degenerate orbital by mixing two atomic orbitals having the same energy. These hybrid orbitals have different shapes and energies from the original atomic orbitals. which is used to explain the bonding and geometries.
This Story also Contains
- PCl5 Hybridization
- $\mathrm{PCl}_5$ Structure
- $\mathrm{PCl}_5$ Lewis Structure
- Some Solved Examples
- Summary
In this article, we will cover the concept of Hybridization. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
PCl5 Hybridization
Compound $\mathrm{PCl}_5$ comprises phosphorus and chlorine in the ratio of P:Cl = 1:5. $\mathrm{PCl}_5$PCl₅ can exist in both solid and gaseous states, but is generally found in gaseous states. In a solid state, it will be in charge form. As we know, phosphorus belongs to the 3rd period in the modern periodic table. In the third period, there are s, p, and s subshells present. So in $\mathrm{PCl}_5$ molecule, hybridization of p will be $s p^3 d$. This hybridization of p in $\mathrm{PCl}_5$ is possible due to the presence of the d orbital in phosphorus. If we talk about energy, then for the 15th group elements like nitrogen, phosphorus (p), arsenic (Ar), antimony (Sb), and bismuth ( Bi) will be in the order:-
-
The energy of 3d ~ energy of 3s ~ energy of 3p, as well as the energy of 3d ~ energy of 4s ~ energy of 4p.
Because of the above reason, hybridization of 3rd-period elements includes 3d, 3p, and 3s or 3d, 4s, and 4p ( as the energy of s and p is equivalent to d) and there is also an energy difference between 3p and 4s orbital, which led to no hybridization of an element with 3p, 3d and 4s.
$\mathrm{PCl}_5$ hybridization
- The hybridization of $\mathrm{PCl}_5$ is sp3d.
$\mathrm{PCl}_5$ shape
- $\mathrm{PCl}_5$ shape is trigonal bipyramidal.
$\mathrm{PCl}_5$ geometry
- The molecular geometry of $\mathrm{PCl}_5$ is a trigonal bipyramid.
Also Read
$\mathrm{PCl}_5$ Structure
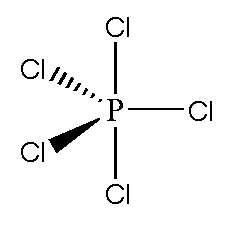
$\mathrm{PCl}_5$ Lewis Structure
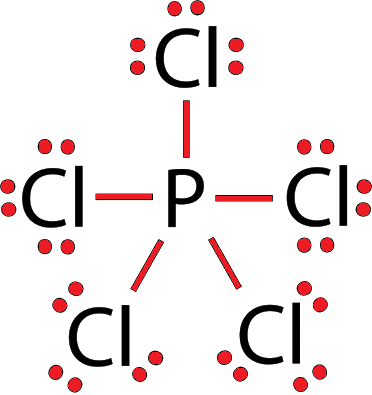
Also read :
- NCERT notes Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 4 Chemical Bonding and Molecular Structure
Some of our important hybridization, including s, p, and d orbital, are given below:-
|
Shape of molecules |
Types of hybridization |
Atomic orbitals |
Example |
|
|
dsp2 |
d+s+p(2) | $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ |
|
|
sp3d |
s+p(3)+d | $\mathrm{PCl}_5$ |
|
|
sp3d |
s+p(3)+d |
BrF5 |
|
|
Sp3d2, d2sp3 |
s+p(3)+d(2), d(2)+s+p(3) |
[CrF6]3–, [Co(NH3)6]3+ |
Some Solved Examples
Question-1 Is $\mathrm{PCl}_6$ hybridization possible?
Ans: $\mathrm{PCl}_6$ hybridization will not be possible because of the presence of only 5 valence electrons in the case of phosphorus. The valency of phosphorus is -3 as it will accept 3 electrons from any other elements to complete its octet and be stable. So $\mathrm{PCl}_6$ is not possible as the formation of $\mathrm{PCl}_6$ phosphorus must contain 6 valence electrons, then it can form 6 bonds, which include coordinating bonds or covalent bonds. But it is not possible in group 3 elements. Because in the modern periodic table, group numbers represent the valency and no. of valence electrons in any element,s we can get through its electronic configuration. And in 3rd period, elements here in the case of phosphorus, there are only 5 valence electrons available.
Question-2 Explain the hybridization involved in phosphorus pentachloride.
Ans: The hybridization involved in P of $\mathrm{PCl}_5$ is 5 sp³d.
Question-3 Explain the formation of $\mathrm{PCl}_5$?
Ans: Here we know that outermost electrons or valence electrons are 5 in the case of phosphorus, and the orbits are 1s, 3p, and 1d i..e.., available for hybridization. And the hybridization of $\mathrm{PCl}_5$ will be sp3d, and we will get a set of 5 hybrid orbitals. So we can represent its hybridization as 5 sp3d, and the no. 5 also represents us about its shape, as its shape has five corners, and i.e, also true for trigonal bipyramidal. And the shape of $\mathrm{PCl}_5$ will be trigonal bipyramidal, we are helpful for VSEPR theory, due to which we can get to know about the molecular shape and its hybridization.
As we know trigonal bipyramidal is a closed figure so there must be bond angles we can discuss and the amazing fact is that not all the bond angles in $\mathrm{PCl}_5$ hybridization i..e.. trigonal bipyramidal is the same, and in this geometry, we can also notice that orbitals of phosphorus i.e. in hybridization and will become sp3d orbital will overlap with p orbital of chlorine and this results in the formation of p-cl bond which counts for 5 in number.
Question-4 PCl₅ shape according to VSEPR theory?
Ans: According to VSEPR theory shape of $\mathrm{PCl}_5$ is trigonal bipyramidal, and its hybridization in the gaseous state is sp³d, but in the solid state, its hybridization will be changed to sp³d² and sp³.
Question-5 How many types of bonds are formed in $\mathrm{PCl}_5$?
Ans: Types of a bond will be 2. There are two types of bonds that will be formed during molecule formation, i.e., $\mathrm{PCl}_5$ formation, and the bonds are equatorial bonds and the other one is axial bonds. The number count for the axial bond is 2, and the number count for the equatorial bond is 3. And both types of bonds are p – cl bonds, indicating that there are 3 p – cl bonds in the equatorial plane and 2 p – cl bonds lie in the axial plane.
Question-6 What are the bond angles of both equatorial bonds and axial bonds?
Ans: • Equatorial bonds, i.e, all 3 p – cl bonds which lie in the same plane. They are arranged by making an angle of 120°.
• Axial bond i.. i.e... all 2 p–Cl bonds in which one p – Cl bond lies above the equatorial surface and the other one p – Cl bond lies below the equatorial plane. These both p – cl bonds make an angle of 90° with p – cl bonds situated in an equatorial plane or equatorial bond. The angle between both p–cl bonds in axial position is 180° as they are situated just opposite to each other
Question-7 Which bond is weaker, equatorial or axial?
Due to repulsive interaction caused by the equatorial bond pair towards the axial bonds, the axial bond pairs are slightly longer to reduce repulsive interaction, but due to increasing its length its strength reduces ( i..e.. length of bond is inversely proportional to the strength of bond ) and so axial bonds are slightly weaker than equatorial bond due to more repulsive interaction from equatorial bond pairs.
Question-8 Hybridization of $\mathrm{PBr}_5$ and $\mathrm{PCl}_5$?
- In a gaseous state, phosphorus of both $\mathrm{PBr}_5$ and $\mathrm{PBr}_5$ exists in sp³d hybridization.
- But in solid state, p of $\mathrm{PBr}_5$ exists in both sp³d² and sp³ hybridization states, while phosphorous, i.e, P of $\mathrm{PBr}_5$, will exist in $\mathrm{sp}^3$ hybridization only.
Question-9 What is the geometry of PCl4+?
- In $\mathrm{PCl}_4^{+}$, there is a 4p–Cl bond and no lone pair.
- So the geometry of $\mathrm{PCl}_4^{+}$ will be tetrahedral.
Question 10:Hybridisation of phosphorus in $\mathrm{PCl}_3$ is
1) (correct) $\mathrm{sp}^3$
2) $\mathrm{sp}^2$
3) sp
4) $s p^3 d$
Solution:
As we have learnt,
Structure of phosphorus trichloride -
Trigonal Pyramidal shape, P is $\mathrm{sp}^3$ hybridised
wherein
.png)
Hybridisation is $\mathrm{sp}^3$
Hence, the answer is the Option (1)
Practice More Questions With the Link Given Below:
Summary
$\mathrm{PBr}_5$ has sp3d hybridization and trigonal pyramidal geometry, and there is no lone pair in $\mathrm{PBr}_5$, so it is Trigonal Pyramidal.
Also read -
