Photoelectric Effect
The photoelectric effect is studied under the context of Atomic structure and the behavior of electrons in atoms when electrons interact with electromagnetic radiations. A lot of experiments were conducted to understand the behavior of atoms. It was one of the experiments conducted in which a metal surface was exposed to light radiation, and it was observed that electrons were emitted from the metal surface. This phenomenon is termed as Photoelectric effect. Quantum mechanics was one of the main experimental evidence of the photoelectric effect. It has shown that light may act in some cases as a wave, and alternatively as a particle (photon), thereby providing evidence for energy quantization.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Spectroscopy:
- Chemical Reactions:
- Photoelectric Effect: Photon Magic
- Some Solved Examples
- Conclusion
In this article, we will cover the concept of the Photoelectric effect. This concept falls under the category of Atomic structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Spectroscopy:
A branch of chemistry dealing with the interaction between matter and radiation is spectroscopy. As such, spectroscopy is relevant in the photoelectric effect, explaining how photons having specific energies are absorbed by atoms and molecules making transitions from one energy level to another.
In particular, the photoelectric effect is used in methods such as Photoelectron Spectroscopy (PES) and X-ray Photoelectron Spectroscopy (XPS) to determine how electron energies are distributed over samples. These methods provide information about the electronic structure and chemical composition of substances.
Chemical Reactions:
Light can initiate photochemical reactions that serve either as reactants or catalysts in some chemical processes. Such reactions involve the emission of light from a molecule after it has absorbed a photon, resulting in rearrangement in its electronic state.
Photoelectric Effect: Photon Magic
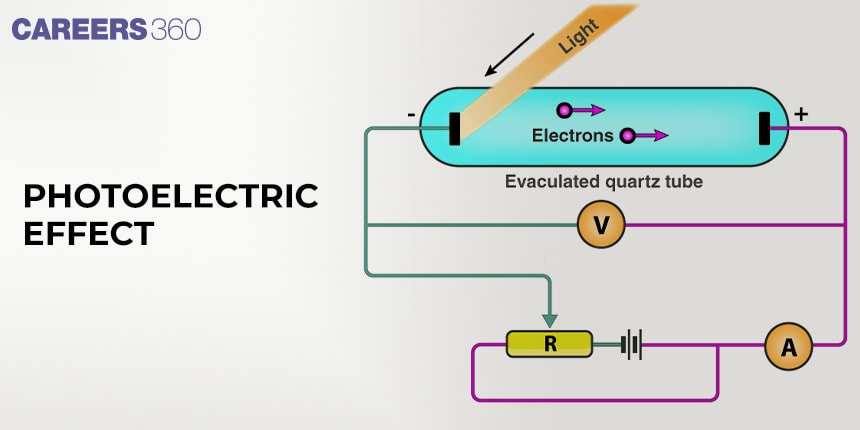
Whenever a metal surface is exposed to light radiation of suitable energy or frequency, it was observed that some electrons get ejected from the metal surface. This phenomenon is called as Photoelectric effect and the ejected electrons are called photoelectrons.

There were certain observations in the photoelectric effect experiment.
(1) There was a requirement of a minimum energy for each metal for the photoelectric effect to occur. This minimum energy is known as work function (W0) and it can be closely associated with the ionization energy of the metal.
- Corresponding to the work function, there is a minimum frequency of light required for the photoelectric effect. This minimum frequency is called the Threshold frequency.
- Corresponding to the work function, there is a maximum wavelength of incident light above which the Photoelectric effect cannot occur. This maximum wavelength is called the Threshold wavelength.
Mathematically, the work function, threshold frequency, and threshold wavelength can be associated as
W0=hν0=hc/λ0
Note: hc is approximately equal to 2 $\times$ 10-25 J-m or 12400 eV-nm. (eV is the energy in electron volts)
(2) The number of electrons ejected is proportional to the intensity (brightness) of light striking the metal but does not depend upon the frequency of light.
(3) There was almost no time lag between the striking of light and the ejection of photoelectrons
(4) The kinetic energy of the ejected electrons (photoelectrons) depends upon the frequency of the light used.
Einstein's photoelectric equation
From the conservation of energy
Ep=W0+KE
∴hν=hν0+1/2mv2
where
m is the mass of the electron
v is the velocity associated with the ejected electron.
h is Planck’s constant.
v is the frequency of the photon,
v0 is the threshold frequency of metal.
(5) The Kinetic energy of an ejected photoelectron is also sometimes associated with Stopping Potential. It is defined as the minimum opposing potential applied due to which the kinetic energy of the electron becomes zero.
1/2mv2=eVs
where,
Vs = Stopping potential
e = Charge on electron
Recommended topic video on(Photoelectric effect)
Some Solved Examples
Example 1:
Light of wavelength λ shines on a metal and emits Y electrons per second of average energy Z. What will happen to Y and Z if the intensity of light is doubled?
1)Y will be doubled and Z will be halved
2)Y will remain the same and Z will be doubled.
3)Both Y and Z will be doubled.
4) (correct)Y will increase but Z will remain the same.
Solution
When intensity is doubled, it means that the brightness of the light is doubled but the energy of the incident beam is kept the same.
So on increasing the number of incident photons, the number of electrons emitted per second will increase but the average energy of photoelectrons emitted remains the same.
Hence, the answer is the option (4).
Example 2:
Ejection of the photoelectron from metal in the photoelectric effect experiment can be stopped by applying 0.5 V when the radiation of 250 nm is used. The work function (eV) of the metal is :
1)4
2) (correct)4.5
3)5
4)5.5
Solution
λ=250 nm=2500
Ep=hc/λ=12400/2500=4.96eV
KE=e×Vs=e×0.5 V=0.5eV
Ep=W0+KE
4.96=W0+0.5
W0=4.46eV≈4.5eV
Hence, the answer is an option (2).
Example 3:
A metal surface is exposed to solar radiation
1) (correct)The emitted electrons have energy less than a maximum value of energy depending upon the frequency of the incident radiation.
2)The emitted electrons have energy less than a maximum value of energy depending upon the intensity of the incident radiation.
3)The emitted electrons have zero energy
4)The emitted electrons have energy equal to the energy of photons of the incident light.
Solution
As we have learned in the photoelectric effect,
Energy of Photon = Work Function +KEmax
Here, the expression KEmax represents the maximum possible Kinetic energy of the emitted photoelectron under ideal conditions i.e. assuming no losses.
This implies that, under real (non-ideal) conditions, the Kinetic energy of the electron will be lesser than the value of KEmax.
It is also to be noted that the intensity affects the number of Photoelectrons emitted but does not affect their energy.
Hence, the answer is the option (1).
Example 4 :
The metal mainly used in devising photoelectric cells is :
1)Na
2)Li
3)Rb
4) (correct)Cs
Solution
Cs are used in photoelectric cells as it has the least ionization energy.
Hence, the answer is the option(4).
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Conclusion
To sum up, the photoelectric effect is a foundation in both physics and chemistry, revealing the dual nature of light and providing profound explanations about electrons’ behaviour in atoms and molecules. Its finding led to the development of quantum mechanics, leading to significant advances in spectroscopy, material science, and energy conversion technologies. By explaining how photons interact with matter, the photoelectric effect still contributes to our knowledge of the tiny world and stimulates scientific progress in many fields. We also learned, In particular, the photoelectric effect is used in methods such as Photoelectron Spectroscopy (PES) and X-ray Photoelectron Spectroscopy (XPS) to determine how electron energies are distributed over samples. These methods provide information about the electronic structure and chemical composition of substances.
Frequently Asked Questions (FAQs)
During the photoelectric effect, the minimum amount of potential energy required to stop the kinetic energy of the elections becomes zero.
About the photoelectric effect, the mass of the electron is directly proportional to the stopping potential.
Ep=W0+KE∴hν=hν0+1/2mv2
The maximum wavelength above which the photoelectric effect does not occur is the Threshold wavelength.
Photoelectron Spectroscopy (PES) and X-ray Photoelectron Spectroscopy (XPS) to determine how electron energies are distributed over samples. These methods provide information about the electronic structure and chemical composition of substances.
Also Read
06 Feb'25 11:21 PM
30 Dec'24 03:07 PM
20 Dec'24 11:57 PM
16 Dec'24 11:39 PM
16 Dec'24 11:27 PM
16 Dec'24 11:16 PM
21 Oct'24 04:01 PM
21 Oct'24 12:25 PM