Baking Soda - Preparation, Uses, Structure, Properties, FAQs
Have you ever wondered what is the use of baking soda? Why is it present in our kitchen? What makes it a key player not just in the kitchen, not just in cleaning, personal care, and even medicine? From helping dough rise to neutralizing odors, baking soda, or sodium bicarbonate, seems to do it all. You will get these answers after reading this article on Baking Soda.
This Story also Contains
- Chemical Name of Baking Soda
- Preparation of Baking Soda
- Properties of Baking Soda
- Structure of Baking Soda
- Baking Soda Uses
- Some Solved Examples

Ever wonder how cakes become fluffy, how biscuits puff up while baking, how stubborn stains are removed from clothes, or how a sour stomach soothens? The only answer to all these is a white powder called Baking soda, also known as Sodium Hydrogen Carbonate. This compound is widely used in industries, households, and laboratories. It reacts with readily available acids like lemon and curd, as it is a mild alkali, and releases carbon dioxide gas. Baking soda is also used in cleansing, fire extinguishers, deodorising, and as medicine. It is a non-toxic and biodegradable compound. It is the basic ingredient of every kitchen.
Chemical Name of Baking Soda
The chemical compound sodium bicarbonate formula, sometimes known as baking soda formula or bicarbonate of baking soda chemical formula, has the formula $\mathrm{NaHCO}_3$. A sodium cation (Na+) and a bicarbonate anion $\left(\mathrm{HCO}_3{ }^{-}\right)$ make up this salt. Sodium bicarbonate is a crystalline white substance that frequently appears as a fine powder. It tastes slightly salty and alkaline, similar to washing soda (sodium carbonate). The chemical name of baking soda is sodium hydrogen carbonate.
Nahcolite is the natural mineral form. It is found dissolved in many mineral springs as a component of the mineral natron. Sodium Bicarbonate's common name is baking soda or the chemical name of baking soda is sodium bicarbonate. Because of its long history and widespread use, baking soda, bread soda, cooking soda, and bicarbonate of soda are all common names for the salt, which can often be purchased beside baking powder in supermarkets.
Preparation of Baking Soda
The Solvay process is used to make sodium bicarbonate as well as sodium carbonate. Carbon dioxide, water, ammonia, as well as concentrated brine solution are employed as raw materials in this process. The important chemical reaction used in the production of baking soda (sodium hydrogen carbonate) and sodium carbonate is:
$\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{NH}_3+\mathrm{NaCl} \rightarrow \mathrm{NaHCO}_3+\mathrm{NH}_4 \mathrm{Cl}$
$2 \mathrm{NaHCO}_3 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
The carbon dioxide produced is recycled to make $\mathrm{NaHCO}_3$.
Read more :
Properties of Baking Soda
- It is non-combustible.
- Powder dust is not as explosive as other types of dust.
- It has a melting point of 500°C and is an odourless white crystalline solid.

|
Related Topics Link, |
|
Sodium bicarbonate |
NaHCO3 |
|
Molar Mass |
84.0066 g/ mol |
|
Boiling Point |
851°C |
|
Melting Point |
50°C |
|
Density |
Solids: 2.20 g/cm3 |
-
It is basic in nature.
$\mathrm{NaHCO}_3+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{CO}_3+\mathrm{NaOH}$
$\mathrm{H}_2 \mathrm{CO}_3 \rightarrow \mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
The pH of baking soda is 8.3.
-
Baking soda is sparingly soluble in water.
-
NaHCO3 crystallizes in a monoclinic crystal lattice.
-
When sodium bicarbonate is heated, it decomposes into sodium carbonate, water, and carbon dioxide gas. At temperatures exceeding 80°C, thermal decomposition occurs.
$2 \mathrm{NaHCO}_3 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}$
-
Baking soda produces carbon dioxide gas when it combines with acids. The following is the chemical reaction that occurs:
$\mathrm{NaHCO}_3+\mathrm{H}^{+} \rightarrow \mathrm{Na}^{+}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
Baking soda is mined as well as manufactured in factories. In its mineral form, it is called nahcolite.
$\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaHCO}_3$
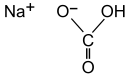
Structure of Baking Soda
-
The chemical formula for sodium bicarbonate is NaHCO3.
-
It is made up of the sodium cation (Na+) and the bicarbonate anion (HCO3-).
-
Its lattice structure is monoclinic.
-
One sodium atom, one carbon atom, one hydrogen atom, and three oxygen atoms make up this compound.
NCERT Chemistry Notes:
Baking Soda Uses
-
Baking soda has antifungal and antibacterial properties, making it an excellent natural solution to a variety of aesthetic problems. It is mildly abrasive, so it will exfoliate the face, feet, hands, and body softly.
-
Baking soda is used as an antacid to treat indigestion and heartburn. Its fast action reduces stomach acid, providing temporary relief. Its alkaline composition helps to reduce indigestion by neutralising excess hydrochloric acid in the stomach.
-
It is a household wonder chemical that is generally used as a cleaning agent, a cooking component, and a disinfectant.
-
It is used to raise flour batter. Baking soda is used to make baking powder, which is made by mixing baking soda with a moderate acid like tartaric acid. In creating bread, cakes, cookies, pancakes, patties, and other baked goods, baking powder is used as a leavening agent (a chemical that releases a gas and causes dough to rise and expand).
-
Baking soda can be used as a natural pesticide in the garden to kill dangerous insects and fungi that attack plants. It can be mixed into soil to raise the pH level and make the soil alkaline. Alkaline soils are preferred by several plants.
Also read -
Some Solved Examples
Question 1: An ingredient of baking powder is:
1) (correct) $\mathrm{NaHCO}_3$
2)$\mathrm{Na}_2 \mathrm{CO}_3$
3) $\mathrm{Na}_2 \mathrm{SO}_4$
4) NaCl
Solution: The Common name of Sodium bicarbonate $\left(\mathrm{NaHCO}_3\right)$ is baking powder.
Hence, the answer is option (1).
Question 2: Solvay's process is used for the preparation of sodium carbonate. Which among the following raw materials are used in Solvay's process?
1) (correct) $\mathrm{CaCO}_3, \mathrm{NH}_3, \mathrm{NaCl}$
2) $\mathrm{NaOH}, \mathrm{CO}_2$
3) $\mathrm{NaCl}, \mathrm{CaCO}_3, \mathrm{C}, \mathrm{H}_2 \mathrm{SO}_4$
4) $\mathrm{NH}_3, \mathrm{H}_2 \mathrm{O}, \mathrm{NaCl}$
Solution:
The raw materials used in solvay's process are: $\mathrm{CaCO}_3, \mathrm{NH}_3, \mathrm{NaCl}$
The following reactions take place during solvay's process.
First of all calcium carbonate is heated and decomposed into calcium oxide and carbon dioxide.
$\mathrm{CaCO}_3 \rightarrow \mathrm{CaO}+\mathrm{CO}_2$
Ammonia reacts with carbon dioxide to form ammonium carbonate.
$2 \mathrm{NH}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow\left(\mathrm{NH}_4\right)_2 \mathrm{CO}_3$
Excess of carbon dioxide is formed to convert ammonium carbonate into ammonium hydrogencarbonate .$\begin{aligned} & \left(\mathrm{NH}_4\right)_2 \mathrm{CO}_3+\mathrm{CaCl}_2 \rightarrow \mathrm{CaCO}_3 \downarrow+2 \mathrm{NH}_4 \mathrm{Cl} \\ & \mathrm{NH}_3+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{NH}_4 \mathrm{HCO}_3\end{aligned}$
Now the ammonium bicarbonate is reacted with sodium chloride. Sodium from sodium chloride replaces ammonia from ammonium bicarbonate and sodium bicarbonate is formed.
$\mathrm{NH}_4 \mathrm{HCO}_3+\mathrm{NaCl} \rightarrow \mathrm{NaHCO}_3+\mathrm{NH}_4 \mathrm{Cl}$
Sodium hydrogencarbonate is obtained from here. It is then further heated to give sodium carbonate i.e. washing soda.$2 \mathrm{NaHCO}_3 \rightarrow \mathrm{Na}_2 \mathrm{CO}_3+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
Hence, the answer is option (1).
Question 3: Which one is known as a fusion mixture?
1)$\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{NaHCO}_3$
2)$\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{NaOH}$
3)$\mathrm{K}_2 \mathrm{CO}_3+\mathrm{K}_2 \mathrm{SO}_4$
4) (correct) $\mathrm{Na}_2 \mathrm{CO}_3+\mathrm{K}_2 \mathrm{CO}_3$
Solution:
Fusion mixture is the mixture of potassium carbonate and sodium carbonate.
The mixture is fused with the organic compounds, and various tests for the group analysis are done using this mixture. It is very much effective for the qualitative analysis for the organic compounds for different groups.
Since Na and K belong to the first group, i.e., the alkali metals, which are the most reactive elements in the periodic table. They have the potential to break the covalent bonds formed between the carbon atom and other atoms like nitrogen, oxygen, sulphur, halides, etc., and after breaking these covalent bonds, ionic bonds are introduced between the respective atoms and the Na or K in the fusion mixture.
Like with the halides, they form the halides and with sulphur, they form the sulphides, etc.
So, a mixture of sodium carbonate and potassium carbonate is called the fusion mixture.
Hence, the answer is option (4).
Practice More Questions With The Link Given Below
Frequently Asked Questions (FAQs)
Baking soda, chemically known as sodium bicarbonate (NaHCO3), is a white crystalline powder. It is typically produced through a process known as the Solvay process, which involves reacting sodium carbonate with carbon dioxide and water.
Yes, baking soda is an excellent natural cleaning agent! Its mild abrasiveness makes it effective for scrubbing surfaces, while its alkaline nature helps neutralize odors and break down dirt and grease. It’s commonly used in kitchens and bathrooms for tasks like cleaning countertops, sinks, and ovens.
Yes, Baking soda is recognized as safe for consumption by food safety authorities around the world. However, it should be used in appropriate amounts, as excessive consumption can lead to health issues, such as metabolic alkalosis or electrolyte imbalances.
Baking soda should be stored in a cool, dry place in an airtight container to keep it fresh and effective. Exposure to moisture or air can cause it to lose potency over time.
Baking soda is primarily used as a leavening agent in baking. When combined with an acid, it releases carbon dioxide gas, which helps baked goods rise and become fluffy.
