Principle of UV Visible Spectroscopy - Explanation, FAQs
Principle of UV Visible Spectroscopy
The principle of UV spectroscopy or the UV-Visible Principle or UV Principle Spectroscopy is based on chemical compounds absorption of ultraviolet or visible light, which results in the formation of different spectra. The interaction of light and matter is the basis of spectroscopy. Excitation and de-excitation occur as matter absorbs light, resulting in the formation of a spectrum.
When matter absorbs ultraviolet light, the electrons inside it become excited. This leads them to move from a ground state (an energy state having a little amount of energy associated with it) to an excited state (an energy state with a relatively large amount of energy). It's worth noting that the difference between the energies of the electron's ground and excited states is always equal to the quantity of ultraviolet or visible energy it absorbs.
UV-visible (UV-Vis) spectroscopy is a widely utilized technique in many fields of science, including bacterial culture, drug identification, and nucleic acid purity checks and quantification, as well as beverage quality control and chemical research. This article will explain how UV-Vis spectroscopy works, how to assess the results, the technique's advantages and disadvantages, and some of its uses. UV spectroscopy Slideshare or UV visible spectroscopy slideshare. would be helpful for knowing the concept better.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is UV-Vis Spectroscopy
UV-Vis spectroscopy is an analytical technique that is used to analyse the wavelengths of UV light absorbed by a sample or transmitted through a sample by comparing using a reference sample. The sample composition provides information about the sample and its concentration.
Because this spectroscopy approach relies on the usage of light, we'll start with light's qualities.
The quantity of energy contained in light is inversely proportional to its wavelength. As a result, shorter light wavelengths carry more energy while longer wavelengths carry less. To promote electrons in a substance to a higher energy state, which we can detect as absorption, a precise quantity of energy is required. In a substance, electrons in different bonding environments require a varied amount of energy to promote them to a higher energy state. This is why different wavelengths of light are absorbed by different things.
The UV visible range from around 380 nm, which we perceive as violet, to 780 nm, which we perceive as red. UV light has wavelengths that are shorter than visible light, up to about 100 nm. As a result, light may be defined by its wavelength, which can be useful in UV-Vis spectroscopy for analyzing or identifying various compounds by locating the exact wavelengths that correlate to maximal absorbance.
UV Vis Spectrophotometer
UV spectroscopy instrumentation is shown below.
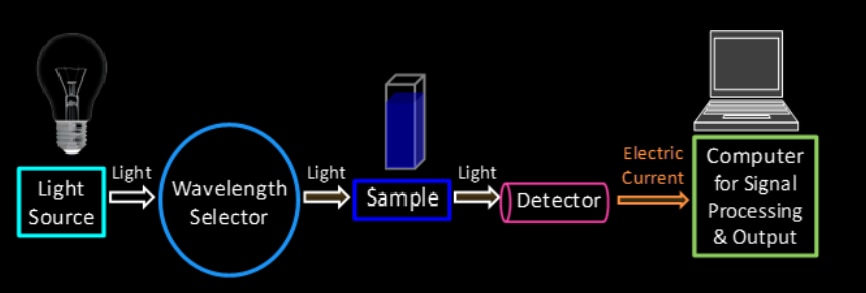
A UV spectrophotometer is the instrument used in ultraviolet–visible spectroscopy. It compares the intensity of light after it passes through a sample (I) to the intensity of light before it goes through a sample ![]() . The absorbance is the ratio of
. The absorbance is the ratio of ![]() which is commonly given as a percentage (%T). The absorbance and A and transmittance is related by the following formula;
which is commonly given as a percentage (%T). The absorbance and A and transmittance is related by the following formula;
A = -log (%T/100%)
The reflectance can also be measured with the UV–visible spectrophotometer. The UV–visible spectrophotometer compares the intensity of light reflected from sample (I) with the light reflected from reference sample![]() . Here the ratio
. Here the ratio ![]() is the reflectance and is represented as a percentage (%R).
is the reflectance and is represented as a percentage (%R).
The essential components of a UV-Vis spectrophotometer instrumentation are a light source, a diffraction grating in a monochromator or prism, and a sample holder.
Read more-
- NCERT solutions for Class 11 Chemistry Chapter 2 Structure of Atom
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 2 Structure of Atom
- NCERT notes Class 11 Chemistry Chapter 2 Structure of Atom
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
