Raoult’s Law
Rault's law is an important principle in the field of chemistry basically physical chemistry it was discovered by the French scientist Francis Marie Raoult in the year 1887. This discovery of Raoult provided a description of how the vapour pressure of the solvent in the solution is related to the concentration of the solute. Raoult's law was discovered during the intense study of colligative properties of solution which mainly depend upon the number of solute particles in the given amount of solvent and they do not depend upon the nature of the solute they only depend upon the number.
This Story also Contains
- Raoult's Law
- Some Solved Examples
- Summary
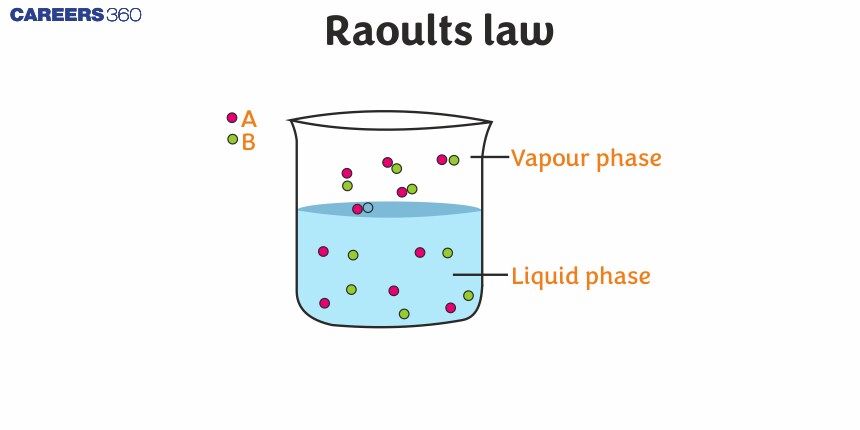
Initially, the results experiment was based on a simple solution and in these experiments he also found out that the vapour pressure of the solvent is directly proportional to the mole fraction of the solute in the solutions. This relationship is the basis of Raoult's law which states that the partial vapour pressure of each volatile compound in the solution is directly proportional to the vapour pressure of the pure component multiplied by the mole fraction in the solution
Raoult's Law
These solutions have vapour pressure less than that predicted by Raoult’s law for the entire range of composition.
This happens when the new solute-solvent interactions are stronger than the interactions in the pure components. Since the newly formed forces are stronger than the existing forces, heat is liberated. Hence, the enthalpy change in the mixing of these solutions is negative i.e. ΔHmix<0.
The change in the volume during the mixing process is positive i.e.ΔVmix<0. For example, if 1 litre solution of each of liquids A and B is mixed, then the solution obtained has a volume lesser than 2 litres.
The entropy of mixing is positive as new interactions are introduced into the solution which increases the randomness of the system hence, The mixing process is spontaneous and hence ΔGmix>0
These solutions have vapour pressure greater than that predicted by Raoult’s law.
PA<PAoXA, PB<PBoXB, PT=PA+PB<PAoXA+PBoXB

Examples of solutions showing negative deviation:
Acetone + Chloroform
Nitric acid HNO3 + water
Acetic acid + pyridine
Phenol + Aniline
Solutions showing positive deviation from Raoult’s law
These solutions have a greater vapour pressure than that predicted by Raoult’s law for the entire composition range.
This happens when the new interactions are weaker than the interactions in the pure component (A-B < A-A or B-B interactions). Since the newly formed forces are weaker than the existing forces, heat has to be supplied to break old bonds and form new ones. Hence, the enthalpy change in the mixing of these solutions is positive i.e. ΔHmix>0.
The change in the volume during the mixing process is positive i.e.ΔVmix>0 For example if 1 litre of solutions each of liquid A and B are mixed, then the solution obtained has a volume greater than 2 litres.
The entropy of mixing is positive as new interactions are introduced into the solution which increases the randomness of the system and hence ΔSmix>0. The mixing process is spontaneous and hence ΔGmix<0
These solutions have vapour pressure greater than that predicted by Raoult’s law.
PA>PAoXA, PB>PBoXB
PT=PA+PB>PAoXA+PBoXB

Examples:
C2H5OH + cyclohexane
Acetone + carbon disulphide
Acetone + benzene
Acetone + Ethyl alcohol
Carbon tetrachloride + chloroform or Toluene
Methyl alcohol + water
Water + Ethyl alcohol
Recommended topic video on ( Raoult’s Law)
Some Solved Examples
Example.1
1. Two liquids A and B on mixing produce a warm solution. Which type of deviation from Raoult's law does it show?
1) (correct)Negative deviation
2)Positive deviation
3)Can't say anything
4)Data insufficient
Solution
Warming up of the solution means that the process of mixing is exothermic, i.e.
This implies that the solution shows a negative deviation.
Hence, the answer is the option (1).
Example.2
2. A solution of sulphuric acid in water exhibits:
1) (correct)Negative deviations from Raoult’s law
2)Positive deviations from Raoult’s law
3)Ideal Properties
4)The applicability of Henry’s law
Solution
There is a hydrogen bond between two molecules of sulphuric acid and between two molecules of water, but there is an ion-dipole interaction between sulphuric acid and water. Ion dipole interaction is stronger than hydrogen bonds. So solution shows a negative deviation from Raoult’s law.
Hence, the answer is the option (1).
Example.3
3. Liquids A and B form an ideal solution in the entire composition range. At 350 K, the vapor pressures of pure A and pure B are7×103 Pa and 12×103 Pa , respectively. The composition of the vapour in equilibrium with a solution containing 40 mol per cent of A at this temperature is :
1)xA=0.37;xB=0.63
2) (correct)xA=0.28;xB=0.72
3)xA=0.4;xB=0.6
4)xA=0.76;xB=0.24
Solution
We know that
ya=PAPtotal =PA0×XAPA0×XA+PB0×XB
yA=7×103×0.47×103×0.4+12×103×0.6yA=2.810=0.28
yB=0.72
Hence, the answer is the option (2).
Example.4
4. The vapour pressure of the solution of two liquids A(Pº = 80 mm) and B(Pº = 120 mm) is found to be 100 mm when XA = 0.4. The result shows that:
1)solution exhibits ideal behaviour
2) The solution shows a positive deviation
3) (correct)solution shows negative deviation
4) The solution will show positive deviations for lower concentrations and negative deviations for higher concentrations
Solution
Negative deviation means lower vapour pressure, it suggests a high boiling point, thus the resultant intermolecular force should be stronger than the individual one.
Hence, the answer is the option (4).
Example.5
5. In mixtures A and B components show negative deviation as
(a) ΔVmix >0
(b) ΔVmix >0
(c) A−B interaction is weaker than A−A and B−B interaction
(d) A−B interaction is stronger than A−A and B−B interaction
The correct statement is/are:
1)(a) only
2)(b) only
3)(a) and (d) only
4) (correct)(b) and (d) only
Solution
Negative deviation means lower vapour pressure, it suggests a high boiling point, thus the resultant intermolecular force should be stronger than the individual one.
Hence, the answer is the option (4).
Summary
Raoult's law is used to describe the relationship between the vapour pressure of solution and the concentration of solute. raoult's law has various benefits in chemistry such that it is used to understand the behaviour of the solution as it predicts how the presence of solutes affects the vapour pressure of the solution which is important for understanding the properties of the solution. Raoults law is also used to determine the molecular weight of the solutions in such a way that the methods used are ebullioscopy and cryoscopy which is used to measure the change in boiling and freezing point.