Redox Reactions - Examples, Types, Applications, Balancing
Redox reaction includes reducing and oxidizing agents which are the main components of this reaction
Oxidizing Agent- The substance that gains electrons and gets reduced. It causes another substance to be oxidized.
Reducing Agent- The substance that loses electrons and gets oxidized. It causes another substance to be reduced.
This Story also Contains
- What Is Redox Reaction
- Redox Reaction:
- Types Of Redox Reaction
- Some Solved Examples
- Summary
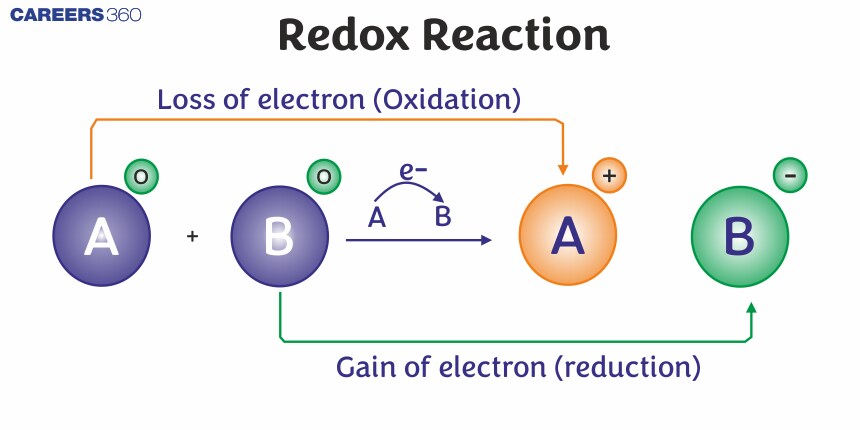
What Is Redox Reaction
A redox (reduction-oxidation) reaction involves the transfer of electrons between two substances. This type of reaction is fundamental in chemistry and is central to many biological and industrial processes.
- Oxidation: The process in which a substance loses electrons. This leads to an increase in the oxidation state of the substance.
- *Reduction: The process in which a substance gains electrons. This leads to a decrease in the oxidation state of the substance.
Redox Reaction:
Any reaction, in which the electrons are exchanged between atoms or ions, represents a simultaneous process of oxidation and reduction is called a Redox Reaction.
A0 + B0 → A+ + B-
Here, A to A+, Loss of electron ( Oxidation )
And B to B-, Gain of electron ( Reduction )
Some examples include:
$\mathrm{Zn}(\mathrm{s})+\mathrm{CuSO}_4(\mathrm{aq}) \rightarrow \mathrm{ZnSO}_4(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$ (Metal displacement)
$\mathrm{Na}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})($ Non - Metal displacement $)$
Some other examples of redox reactions in which oxidation and reduction are happening simultaneously.
$\mathrm{P}_4(\mathrm{~s})+3 \mathrm{OH}^{-}(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{PH}_3(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{PO}_2^{-}$
Types Of Redox Reaction
The different types of redox reactions are:
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reactions
Decomposition Reaction
This is the reaction that involves the breakdown of a compound into different compounds. Some examples of this type of reaction are:
$2 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \xrightarrow{\Delta} 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})$$2 \mathrm{KClO}_3(\mathrm{~s}) \xrightarrow{\Delta} 2 \mathrm{KCl}(\mathrm{s})+3 \mathrm{O}_2(\mathrm{~g})$
This must be noted here that all decomposition reactions are not redox reactions. For example, the decomposition of calcium carbonate is not a redox reaction.$\mathrm{CaCO}_3(\mathrm{~s}) \xrightarrow{\Delta} \mathrm{CaO}(\mathrm{s})+\mathrm{CO}_2(\mathrm{~g})$
Combination Reaction
These types of reactions are the opposite of decomposition reactions and hence involve the combination of two species to form a single compound. Some examples include:
$\mathrm{C}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \xrightarrow{\Delta} \mathrm{CO}_2(\mathrm{~g})$$3 M g(s)+N_2(g) \rightarrow M g_3 N_2(s)$
Displacement Reaction
Displacement reactions, also known as replacement reactions, involve compounds and the replacement of elements. They occur as single and double replacement reactions. In other words, in these type of reactions, an atom or an ion in a compound is substituted by another element.
Some examples include:
$\mathrm{Zn}(\mathrm{s})+\mathrm{CuSO}_4(\mathrm{aq}) \rightarrow \mathrm{ZnSO}_4(\mathrm{aq})+\mathrm{Cu}(\mathrm{s})$ (Metal displacement)
$\mathrm{Na}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{NaOH}(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})(\mathrm{Non}-$ Metal displacement
Disproportionation Reactions
Disproportionation reactions are those reactions in which a single element in one oxidation state is simultaneously oxidized and reduced. Some examples include:
$\mathrm{P}_4(\mathrm{~s})+3 \mathrm{OH}^{-}(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{PH}_3(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{PO}_2^{-}$$\mathrm{S}_8(\mathrm{~s})+12 \mathrm{OH}^{-}(\mathrm{aq}) \rightarrow 4 \mathrm{~S}^{2-}(\mathrm{aq})+2 \mathrm{~S}_2 \mathrm{O}_3^{2-}(\mathrm{aq})+6 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
Recommended topic video on(Redox Reactions)
Some Solved Examples
Example.1
1. Which of the following is a redox reaction?
1)$\mathrm{NaCl}+\mathrm{KNO}_3 \rightarrow \mathrm{NaNO}_3+\mathrm{KCl}$
2)$\mathrm{CaC}_2 \mathrm{O}_4+2 \mathrm{HCl} \rightarrow \mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4$
3)$\mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{NH}_4 \mathrm{Cl} \rightarrow \mathrm{MgCl}_2+2 \mathrm{NH}_4 \mathrm{OH}$
4) (correct)$\mathrm{Zn}+2 \mathrm{AgCN} \rightarrow 2 \mathrm{Ag}+\mathrm{Zn}(\mathrm{CN})_2$
Solution
In a Redox Reaction, the oxidation states of the constituents should change.
Keeping that in mind, let us look at each reaction:
$\stackrel{+1-1}{\mathrm{NaCl}}+\stackrel{+1+5-2}{\mathrm{KNN}} \stackrel{+}{\mathrm{N}}_3 \rightarrow \stackrel{+1+5-2}{\mathrm{Na}} \mathrm{N}_3+\stackrel{+1-1}{\mathrm{KCl}}$
There is no change in the oxidation states.
$\mathrm{CaC}_2 \mathrm{O}_4+2 \mathrm{HCl} \rightarrow \mathrm{CaCl}_2+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4$
There is no change in the oxidation states.
$\mathrm{Mg}(\mathrm{OH})_2+2 \mathrm{NH}_4 \mathrm{Cl} \rightarrow \mathrm{MgCl}_2+2 \mathrm{~N} \mathrm{H}_4 \mathrm{OH}$
There is no change in the oxidation states.
Finally,
$\stackrel{0}{\mathrm{Zn}}+2 \stackrel{+1}{\mathrm{Ag}} \stackrel{+2-3}{\mathrm{~N}} \rightarrow 2 \stackrel{0}{\mathrm{Ag}}+\stackrel{+2}{\mathrm{Zn}}(\stackrel{+2-3}{\mathrm{C}})_2$
There is a change in the oxidation states of the constituents.
The last reaction is redox.
The oxidation states show a change only in reaction (d).
Hence, the answer is the option (4).
Example.2
2. Which of the following reactions is an example of a redox reaction?
1)XeF6 + H2O → XeOF4 + 2HF
2)XeF6 + 2H2O → XeO2F2 + 4HF
3) (correct)XeF4 + O2F2 → XeF6 + O2
4)XeF2 + PF5 → [XeF]+ PF6−
Solution
In the given reactions:
$\mathrm{XeF}_6+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{XeOF}_4+2 \mathrm{HF}$
The oxidation states are:
$\mathrm{XeF}_6+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{XeOF}_4+2 \mathrm{HF}$
There is no change in the oxidation states of any of the reactants.
The above is not a redox reaction.
Similarly, $\mathrm{XeF}_6+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{XeO}_2 \mathrm{~F}_2+4 \mathrm{HF}$
$\therefore$ Above is not a redox reaction
In the reaction,
(+2) (-1) (+5) (-1) (+2) (-1) (+5) (-1)
$X e F_2+P F_5 \rightarrow[X e F]^{+}\left[P F_6\right]$
$\therefore$ Above is not a redox reaction
Now,
+4 -1 +1 -1 +6 -1 0
$X e F_4+O_2 F_2 \rightarrow X e F_6+O_1$
The oxidation state of Xe changed from +4 to +6 and that of oxygen changed from (+1) to 0
This is an example of a redox reaction.
Hence, the answer is an option (3).
Example.3
3. In the following reaction which species undergoes reduction?
$\left.2 \mathrm{Na}_{(} \mathrm{s}\right)+\mathrm{H}_{2(g)} \rightarrow 2 \mathrm{NaH}_{(\mathrm{s}}$
1)Na
2) (correct)H
3)NaH
4)Not a redox reaction.
Solution
Redox Reaction -
Redox reaction is a class of reactions in which oxidation and reduction reactions occur simultaneously.
Since hydrogen is more electronegative than sodium, so hydrogen undergoes reduction.
Hence, the answer is the option (2).
Example.4
4. In the following reaction, which species undergoes oxidation?
$\mathrm{Cl}_{2(g)}+3 \mathrm{~F}_{2(g)} \rightarrow 2 \mathrm{ClF}_{3(g)}$
1) (correct)Cl
2)F
3)Neither
4)The reaction is not redox
Solution
Redox Reaction -
Redox reaction is a class of reactions in which oxidation and reduction reactions occur simultaneously.
Since fluorine is more electronegative than chlorine, so chlorine undergoes oxidation.
Hence, the answer is the option (1).
Example.5
5. On heating $\mathrm{KClO}_2$ it gives KCl and O2 This reaction is known as
1)Oxidation
2)Reduction
3)Disproportionation
4) (correct)Redox
Solution
Redox reaction is a class of reactions in which oxidation and reduction reactions occur simultaneously.
In the reaction :
$2 \mathrm{KClO}_3 \rightarrow 2 \mathrm{KCl}+3 \mathrm{O}_2$
Cl is reduced from +5 to -1 oxidation state and O is oxidized from -2 to 0 oxidation state.
So, it is an example of a redox reaction.
Hence, the answer is the option (4).
Summary
Redox reactions are essential in mining for extracting metals, processing ores, managing environmental impacts, and optimizing energy use. They are fundamental to both the technical and environmental aspects of mining operations. Redox reactions are often exothermic or endothermic, impacting the energy requirements of mining processes. Redox reactions help control the acidity or alkalinity of the solution, optimizing the extraction and concentration of desired metals.