Reverse Osmosis: Definition, Process, and Uses
The principle of osmosis was first discovered by the French scientist Jean Antoine Nollet. During his study, he observed that water can pass through the membrane namely the semipermeable membrane. The idea of reverse osmosis began to take shape in the 1950s with the development of synthetic membranes and it happened because of researchers aiming to address the water quality and also due to water scarcity.
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Osmosis
- Reverse Osmosis
- Some Solved Example
- Summary
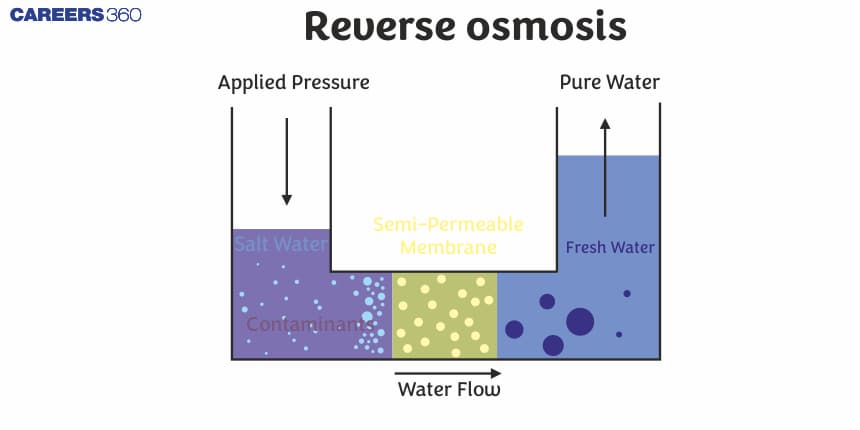
Reverse osmosis is basically a process that is used to remove a wide range of contaminants which include, salt, bacteria, and other impurities present in the water or any chemical that is dissolved in the water. It is an important source for the region where the resources of fresh water are limited. Reverse osmosis is also used by the municipalities to improve the quality of water for further supply so that water reaches the people and is pure.
Osmosis
Osmosis: It is the flow of solvent molecules from a solution of low concentration to a solution of higher concentration when they are separated by a semi-permeable membrane(SPM), the concentration obviously being defined with respect to the solute.
Semi-permeable membrane consists of a network of submicroscopic pores or holes. The pore size is such that the smaller solvent molecules can move across the membrane while the movement of larger solute molecules is hindered by the smaller pores of the SPM.
There are many phenomena which include the process of osmosis that we observe in daily lives. For example, raw mangoes shrivel when pickled in brine (saltwater); wilted flowers revive when placed in freshwater, blood cells collapse when suspended in saline water, etc.
Assume that only solvent molecules can pass through these semipermeable membranes. If this membrane is placed between the solvent and solution as shown in the figure given below, the solvent molecules will flow through the membrane from pure solvent to the solution. This process of flow of the solvent is called osmosis.

Reverse Osmosis
The direction of osmosis can be reversed if a pressure larger than the osmotic pressure is applied to the solution side. That is, now the pure solvent flows out of the solution through the semi-permeable membrane. This phenomenon is called reverse osmosis and is of great practical utility. Reverse osmosis is used in the desalination of seawater. A schematic setup for the process is shown in the Figure given below. When pressure more than the osmotic pressure is applied, pure water is squeezed out of the seawater through the membrane. A variety of polymer membranes are available for this purpose.

The pressure required for reverse osmosis is quite high. A workable porous membrane is a film of cellulose acetate placed over suitable support. Cellulose acetate is permeable to water but impermeable to impurities and ions present in seawater. These days many countries use desalination plants to meet their potable water requirements.
Recommended topic video on (Reverse osmosis)
Some Solved Example
Example.1
1. RO membranes are made of:
1)Plastic
2)Cotton
3)Silk
4) (correct)Polymer
Solution
The highest recommended applied pressure of commercial membranes presently available is 7.0 Mpa; beyond which compaction will start to occur because RO membranes are made of polymers.
Hence, the answer is the option (4).
Example.2
2. As water viscosity lowers, the water flux of RO membranes?
1)Fluctuates
2)Decreases
3) (correct)Increases
4)None of the above
Solution
When the water viscosity is lowered, the water flux of RO membranes increases and the water viscosity is lowered by an increase in temperature. Thus, the higher the temperature better the flux.
Hence, the answer is the option (3).
Example.3
3. What is the flux considered while designing a reverse osmosis system for treating bore well water?
1)10-15 LMH
2)40-50 LMH
3) (correct)20-30 LMH
4)60-70 LMH
Solution
The flux increases with an increase in pressure. It also increases with increased temperature. In the case of bore well water, the flux considered is 20-30 LMH.
Hence, the answer is the option (3).
Example.4
4. The size of a raw mango shrinks to a much smaller size when kept in a concentrated salt solution which one of the following processes can explain this?
1)Reverse osmosis
2)Diffusion
3) (correct)Osmosis
4)Dialysis
Solution
The size of the mango shrinks when it is kept in a concentrated salt solution. This is due to osmosis in which the water molecules move out from the mango into the salt solution causing the mango to shrink.
Hence, the answer is the option (3).
Example.5
5. What is the maximum acceptable limit of temperature(in oC) for RO?
(Response should be an integer value)
1) (correct)40
2)30
3)15
4)50
Solution
To reduce the effects of temperature to a minimum, the acceptable upper limit is 40. RO systems operate at 25oC usually.
Hence, the answer is the option (1).
Example.6
6.Match List I and List II

Choose the correct answer from the options given below :
1)A-I, B-III, C-IV, D-II
2) (correct)A-III, B-I, C-IV, D-II
3)A-III, B-I, C-II, D-IV
4)A-I, B-III, C-II, D-IV
Solution
(i) Electro osmosis: When the movement of colloidal particles is prevented by some suitable means (porous diaphragm or semi-permeable membranes), it is observed that the D.M. begins to move in an electric field. This phenomenon is termed electrosmosis.
(ii) Solvent molecules pass through a semi-permeable membrane towards the solvent side is termed reverse osmosis.
(iii) When an electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards move towards one or the other electrode. The movement of colloidal particles under an applied electric potential is called electrophoresis.
(iv) Solvent molecules pass through a semipermeable membrane towards the solution side is termed as osmosis.
Summary
It has been proved that the phenomenon of reverse osmosis is a highly effective and very versatile technology for the purification of water and has various significant applications in other fields. In this process, water is forced to pass through the semi-permeable membrane that removes all the contaminants of water which results in purified water. The water produced as a result of reverse osmosis is high quality as it has a desirable taste and is free from undesirable taste, odor, and pollutants. As reverse osmosis is very effective, it is also energy intensive and it can be costly in terms of its maintenance and operation. In this process, the semi-permeable membrane needs to be changed frequently because the membrane is the most working part of this process.
Also Read
19 Feb'25 06:42 PM
19 Feb'25 06:38 PM
19 Feb'25 06:35 PM
19 Feb'25 06:29 PM
19 Feb'25 06:23 PM
19 Feb'25 05:11 PM
25 Oct'24 05:23 PM
18 Oct'24 10:46 AM
10 Oct'24 12:09 PM
10 Oct'24 12:06 PM