Rutherford Atomic Model And Its Limitations
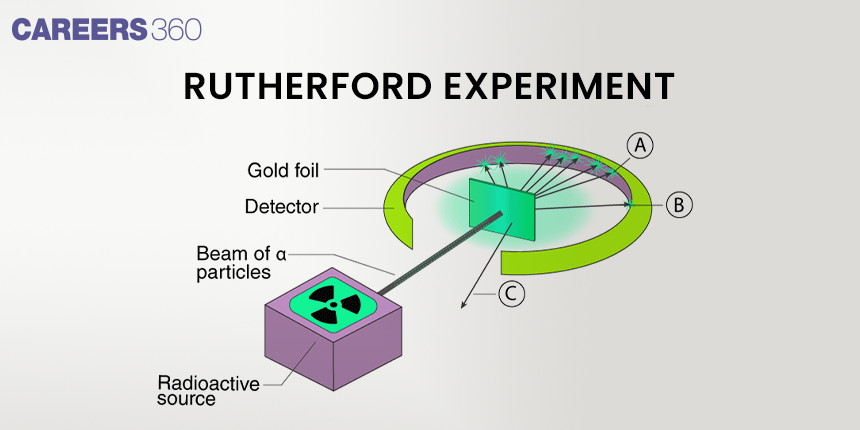
The famous Rutherford's Alpha Scattering Experiment, which clarifies the fundamentals of atomic structure, was conducted by Ernest Rutherford. Using a radioactive source, Rutherford directed high-energy streams of α-particles towards a 100 nm-thick gold sheet. We can learn about the atomic structure from the deflection of these alpha particles. To find out how the alpha particles were deflecting the thin gold foil, he placed a fluorescent zinc sulphide screen around it.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Rutherford's Alpha Adventure: Unveiling What's Inside the Atom?
- Observations of the Rutherford's Experiment:
- Rutherford's Atomic Model's Conclusion:
- Limitations of the Rutherford's Model:
- Solved Examples Based On Rutherford's α Scattering Experiment
- Conclusion
In this article, we will cover the concept of Rutherford's Alpha particle Experiment, observations including the conclusion based on the experiment. This concept falls under the broader category of Atomic structure, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more.
Let us study in detail the famous experiment conducted by Rutherford to gain insights into this topic and solve a few related problems.
Rutherford's Alpha Adventure: Unveiling What's Inside the Atom?
A stream of high-energy α–particles from a radioactive source was directed at a thin foil (thickness ∼ 100 nm) of gold metal. The thin gold foil had a circular fluorescent zinc sulphide screen around it. Whenever α–particles struck the screen, a tiny flash of light was produced at that point

Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Observations of the Rutherford's Experiment:
- Most of the α–particles passed through the gold foil undeflected indicating that most of the atom is space.
- A small fraction of the α–particles was deflected by small angles indicating that the positive charge is concentrated in a minimal volume.
- Very few α–particles (∼1 in 20,000) bounced back, that is, were deflected by nearly 180° thus confirming the size of the nucleus to be very small.
Rutherford's Atomic Model's Conclusion:
Most of the part of the atom is empty and the atom is spherical.
Each atom consists of a small, heavy, positively charged portion located at the centre, known as the nucleus.
All positive charge of atoms (i.e. protons) are present in the nucleus and electrons move around the nucleus in circular orbits.
Electrons and the nucleus are held together by electrostatic forces of attraction.
Limitations of the Rutherford's Model:
According to Maxwell's theory, a moving charged particle under acceleration radiates energy and thus the electron must spiral into the nucleus, but that does not occur. The stability of the atom was not explained by Rutherford's model.
Rutherford's model does not provide any information about the position of the electron or its energy.
Recommended topic video on (Rutherford atomic model and its limitations)
Solved Examples Based On Rutherford's α Scattering Experiment
Example 1: If the Thomson model of the atom was correct, then the result of Rutherford's gold foil experiment would have been :
1) All of the α-particles pass through the gold foil without a decrease in speed.
2) α-Particles are deflected over a wide range of angles.
3) All α-particles get bounced back by 180∘
4) (correct) α-Particles pass through the gold foil deflected by small angles and with reduced speed.
Solution
Thomson's model is similar to a Plum pudding model in which the electrons are embedded in a positively charged sphere.
If this model were correct, Rutherford's gold foil experiment would have observed the alpha particles pass through the gold foil deflected by small angles and with reduced speed.
Hence, the answer is the option (4).
Example 2: Assertion: Rutherford's atomic model failed to explain the stability of an atom.
Reason: According to Rutherford's atomic model, electrons revolve around the nucleus in circular orbits, but such an arrangement would lead to the acceleration of electrons and ultimately cause the atom to collapse.
(1) (correct) Both assertion and reason are true, and the reason is the correct explanation of the assertion.
(2) Both assertion and reason are true, but the reason is not the correct explanation of the assertion.
(3) The assertion is true, but the reason is false.
(4) The assertion is false, but the reason is true.
Solution
According to Rutherford's atomic model, the electrons revolve around the nucleus in circular orbits, similar to planets orbiting around the sun. However, the model could not explain why the electrons did not emit energy continuously and eventually fell into the nucleus. In classical mechanics, any charged particle undergoing acceleration emits radiation, which causes the particle to lose energy, ultimately causing it to spiral into the nucleus. This limitation was resolved by the development of quantum mechanics, which explained that the electrons exist in discrete energy levels and not in fixed orbits.
Hence, the answer is the option (2).
Example 3: What are the limitations of Rutherford's atomic model?
1) It failed to explain the stability of an atom.
2) It could not explain the spectra of atoms with more than one electron.
3) It did not provide any information about the arrangement of electrons in the atom.
4) All of the above.
Solution
Correct option d) All of the above.
Rutherford's atomic model was a significant improvement over the previous atomic models, but it had some limitations. Firstly, it could not explain the stability of an atom. According to classical mechanics, any charged particle undergoing acceleration emits radiation, which causes the particle to lose energy, ultimately causing it to spiral into the nucleus. Secondly, the model could not explain the spectra of atoms with more than one electron. Lastly, the model did not provide any information about the arrangement of electrons in the atom. These limitations were later resolved by the development of quantum mechanics.
Hence, the answer is the option (4).
Conclusion
Based on his findings, Rutherford postulated the atomic structure of the elements. Rutherford's atomic model states that: A positively charged particle was concentrated in a very small volume, which also contained the majority of an atom's mass. He referred to this as an atom's nucleus. Rutherford asserted that atom nuclei are surrounded by negatively charged electrons. The electron that envelops the nucleus travels quickly in a circular pattern. He gave these circular routes names for their orbits.
Also check-
Frequently Asked Questions (FAQs)
The particle which has no charge and has a mass nearly equal to that of a proton is a neutron, and it is present in the nucleus of the atom.
Ernest Rutherford's 1909 experiment on the scattering of alpha particles.
The purpose of this experiment was to determine the atom's structure.
Although Rutherford's experiment has potential implications in the present era, it also broadened the inventor's perspective on adjacent subjects including nuclear physics, particle physics, and material science. A device called a particle accelerator, which is based on this experiment, is employed in many different types of research.
A thin gold sheet for the experiment, a 100 nm thin sheet, was used.
Also Read
06 Feb'25 11:29 PM
06 Feb'25 11:19 PM
20 Oct'24 05:49 PM
30 Sep'24 12:12 PM
30 Sep'24 11:43 AM
30 Sep'24 11:16 AM
18 Sep'24 10:15 AM
16 Sep'24 03:04 PM