Schiff Reagent and Test - Composition, Preparation, Structure, Reaction, FAQs
In this article we will be discussing Schiff's reagent, Schiff test, Schiff reagent formula, Schiff reagent test, Schiff reagent preparation, Rosaline structure and Schiff’s test for aldehydes. The Schiff reagent test is a test performed to detect the presence of aldehydes in a given compound. This test is named after Hugo Schiff; a scientist who developed this test.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Schiff Test
- Schiff Reagent
- Schiff Reagent Composition
- Schiff Reagent Preparation
- Rosaniline Structure
- Schiff test reaction
Schiff Test
This qualitative test for aldehydes is performed using a Schiff reagent. The sample that needs to be examined is combined with Schiff reagent. If aldehyde is present, a characteristic magenta-pink color is obtained.
Any compound with an aldehydic -CHO group will show a positive Schiff test. Example: Human skin is stained due to the presence of aldehydes in the tissues of the skin. Although, sometimes aliphatic ketones might show positive results the overall process for colorization is slow, and takes time for the pink color to emerge. Aromatic aldehyde reacts similarly as aliphatic ketones react with Schiff reagent but aromatic ketones do not produce color in the presence of Schiff reagent.
Schiff Reagent
Schiff reagent is a product of formulation of certain dyes such as fuchsin and sodium bisulphate which chemically react with aldehydes to form a bright pink product. Schiff reagent is sometimes referred to as leucofuchsin. The term ‘leuco’ means white or absence of color which is termed due to a very pale yellow color or nearly colorless solution. The fuchsin dye is decolorized by the addition of sulfurous acid (or its conjugate base bisulfate) due to which it is also referred to as fuchsin-sulfurous acid. Fuchsin dye is also known as rosaniline hydrochloride and is marked by its magenta color.
Schiff Reagent Composition
The Schiff reagent is prepared by using fuchsin (<1%) dye in water (>98%) combined with sodium bisulfite (<1%) dissolved in a solution of hydrochloric acid (<1%). The solution is shaken at intervals followed by decolorization with charcoal. The mixture is then filtered. Fresh activated charcoal must be used to ensure the formation of a perfectly colorless solution. If the solution is not colorless, the solution is refiltered.
Also Read:
Schiff Reagent Preparation
The precise steps of preparation are stated below:
- In 900ml boiling water, dissolve 5g basic fuchsin.
- Cool down the solution until it reaches around 50°C temperature.
- Slowly add 100ml of 1M HCl to the dilute solution of fuchsin.
- Again cool down the solution to 25°C.
- To the cooled solution add 10g K2S2O5.
- Shake the above solution for 3 minutes and leave the solution mixture to incubate in a dark room for 24 hours.
- After incubation and resting, add 5g of fine activated charcoal to the reaction mixture.
- Shake the solution for 3 minutes and filter.
- Crystal clear solution must be obtained or else refilteration and retreatment must be done.
- Store the prepared solution at 4°C in a bottle covered with foil. Schiff reagent precipitates a white crystalline substance if not stored properly. Thus, fresh batches at intervals of 2-3 weeks must be prepared to ensure accurate results.
| Related topics link, |
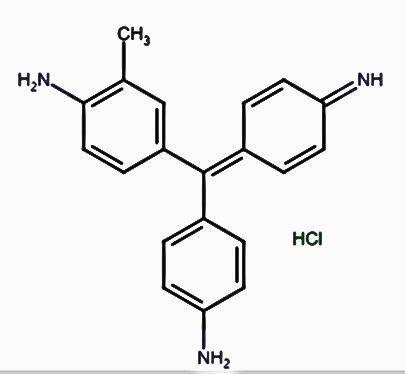
Rosaniline Structure
Rosaniline hydrochloride or fuchsin is a pinkish magenta color dye used in Schiff reagent to detect the presence of aldehydes. In Schiff reagent, it is decolorized by sulfurous acid to form a colorless solution.
Schiff reagent formula: Schiff reagent’s molecular formula is C20H19N3·HCl.
Schiff test reaction
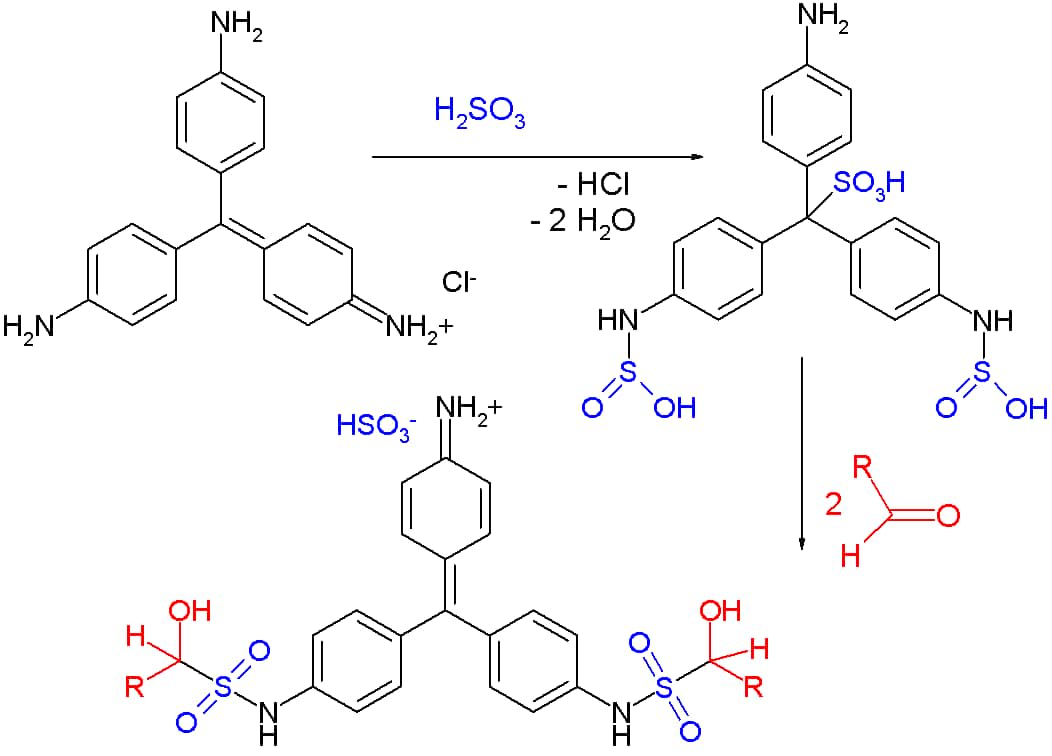
The reaction is initiated when rosaniline hydrochloride or fuchsin with a characteristic magenta color is decolorized by adding sulfurous acid H2SO3. The decolorization is due to distortion of the chromophore (chromophore is a term used to define atoms or groups of atoms that are responsible for the presence of color in a compound) by the addition of sulphonic acid -SO3H group to the central carbon resulting in loss of quinoid ring.
This is known as the Schiff reagent. When this new sulphonic acid substituted compound or Schiff reagent reacts with two molecules of aldehydes, condensation takes place which reforms the chromophore and restores the quinoid ring. Thus, a pink-magenta color is reproduced indicating the presence of aldehydes.
Also, check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Schiff reagent is a reagent used in Schiff’s test for aldehydes. Schiff reagent gives pink colour with aldehydes. It is composed of rosaniline hydrochloride or fuchsin and sulphurous acid.
CH3CHO
C6H5COCH2.CH3
CH3COCH3
HCHO
Ans. B) C6H5COCH2.CH3 does not react with NaHSO3.
As discussed in the beginning, aromatic ketones do not react with Schiff reagent as they are less reactive than aliphatic ketones and both aromatic aldehydes and aliphatic aldehydes.
Fehling’s reagent
Tollen’s reagent
Schiff reagent
Grignard reagent
Ans. D) Grignard reagent
Grignard reagent can react efficiently with acetaldehyde to give secondary and with acetone to give tertiary alcohols.
Although acetone is an aliphatic ketone, it gives a very slow reaction with Schiff reagent. Thus, making it a not-so-good reagent for detecting ketones.
Tollen’s reagent, Fehling’s solution and Schiff reagent are effective for detection of aldehydes only as they are only reduced by aldehydes.
Fehling’s solution
Tollen’s reagent
Schiff reagent
Benedict’s solution
Ans. b) Tollen’s reagent.
Tollen’s reagent is a powerful reagent which can be easily reduced by an aromatic aldehyde like Benzaldehyde and gives silver.
Fehling’s solution and Schiff reagent are weak reagents which cannot be reduced by aromatic aldehydes while Benedict’s solution is used for detecting sugars.
Aromatic aldehydes give a very slow reaction with Schiff reagent as discussed previously.
No, Benzaldehyde does not give Fehling test due to absence of alpha hydrogen and thus cannot form enolate.
Iodoform test
Fehling’s test
Lucas test
Tollen’s test
Ans. Acetaldehyde cannot give c) Lucas test.
Lucas test is a test performed to separate alcohols. The Iodoform test, Fehling’s test and Tollen’s test are identifying tests for aldehydes.
One primary OH and four secondary OH groups
two primary OH and three secondary OH groups
One secondary OH and four primary OH groups
three primary OH and two secondary OH groups
Ans. A) One primary OH and four secondary OH groups.
Schiff test is a qualitative test for aldehydes and is performed using Schiff reagent. The sample that needs to be examined is combined with Schiff reagent. If aldehyde is present, characteristic magenta-pink color is obtained. Any compound with an aldehydic -CHO group will show positive Schiff test.
Schiff reagent is prepared by using fuchsin (<1%) dye in water (>98%) combined with sodium bisulfite (<1%) dissolved in solution of hydrochloric acid (<1%).
Fehling Solution is a chemical test used to distinguish between acetaldehyde and benzaldehyde.
Benzaldehyde does not give any reaction with fehling solution while acetaldehyde gives red colored precipitate with Fehling solution.
Both Schiff base and Schiff reagent are named after Hugo Schiff. Both terms are used for naming a group of particular organic compounds. Schiff base refers to secondary ketimines or secondary aldimines while Schiff’s reagent is a reagent used to test for identification of aldehydes (and some ketones).
Also Read
16 Dec'24 11:34 PM
16 Dec'24 11:32 PM
09 Dec'24 11:34 AM
09 Dec'24 11:33 AM
09 Dec'24 11:14 AM
12 Nov'24 12:07 PM
19 Oct'24 02:52 PM
30 Sep'24 11:42 AM