Sn2 Reaction Mechanism - Examples, Factors, Reaction Rate, FAQs
SN2 Reaction
Substitution nucleophilic bimolecular reaction is a type of reaction mechanism commonly used in organic compounds. It is also a type of nucleophilic substitution reaction. The bimolecular in the name suggests that there are two species involved in the rate-determining step that is the slow step. SN1 is another type of nucleophilic substitution reaction. SN2 reaction also given the name interchange mechanism and associative substitution. SN2 reaction is a single-step reaction in which a bond is broken and a new bond is formed suddenly.
The two stand for bimolecular which means there are 2 components included in the substitution reaction. In this reaction the nucleophilic substitution of a leaving group takes place and the leaving group generally used is halide groups or other electron-withdrawing groups and a new compound is formed after the reaction.
Also read -
What is SN2 Reaction Mechanism?
The SN2 reaction involves the attack of nucleophile from the backside of the carbon skeleton so there will be an inversion of configuration that is the stereochemistry just becoming opposite to that originally present. So, for this reaction, the role of the steric effect is more. It is also the best example of a stereospecific reaction.
A bimolecular nucleophilic substitution reaction takes place in a single step so it is also called a concerted reaction. Here the bond breaking between the electrophilic carbon and the leaving group, and bond making between the nucleophile and electrophile carbon occurs at the same time. So the number of steps in SN2 is one. The leaving group most commonly used is halides.
SN2 Mechanism
The reaction mechanism involves the formation of a single transition state. The bond breaking occurs at the C-X bond and the carbon present is mostly aliphatic. After the breaking of the C-X bond nucleophile attacks and a new bond, C-Nu is formed. Where Nu represents nucleophile, a nucleophile is a species that is rich in electrons and has a tendency to attack electrophiles that are electron-deficient species. In the formation of the C-Nu bond, there is an intermediate state where the carbon is pentacoordinate. And after its formation, the leaving group leaves, and a new bond C-Nu is formed. All these reactions are taking place spontaneously.
SN2 Reaction Examples:
SN2 reaction examples are described in this portion. The following reaction shows the attack of the halogen Br- which is the nucleophile on the carbon atom attached to a leaving group that is Cl. And correspondingly a pentacoordinate transition state is formed and is short-lived. After the formation of the transition state, it spontaneously dissociates to form the product with the breaking of the bond between C-Cl.
SN2 The reaction mechanism in chloropropane using bromine as a nucleophile is shown below. And the stereochemistry of SN2 reaction is shown below.
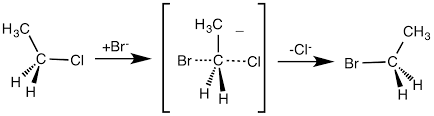
In the ethyl chloride, the attack of nucleophile is from the backside so the stereochemistry gets inverted. This inversion in configuration is called Walden Inversion. Since it involves the attack from the backside, the backside should not be sterically hindered. That is primary and secondary carbon is more preferred on SN2 reactions. If bulky substituents or tertiary carbon are present the reaction will not proceed. Tertiary carbon is more preferred in SN1 reaction.
Some other Examples of SN2 Nucleophilic Substitution
The nucleophilic substitution reaction taking place on 2-Bromobutane with the nucleophile OH- is shown below. In the first step, an attack takes place from the backside and there is a formation of transition state which then suddenly dissociates to form the product. Here the leaving group is Br-.
The SN2 reaction mechanism taking place in 2-bromopropane using hydroxyl ion as a nucleophile is shown below.
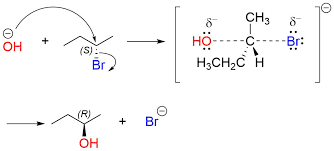
Factors Affecting SN2 Reaction Mechanism
Effect of substrate
The substrate present should be free from bulky groups since the attack is taking place from the backside. And also the breaking and making of bonds are taking place in a single step the selection of substrate is very important. The carbon present should not be tertiary also.
Effect of nucleophile
The selection of nucleophiles has also a very good role in the reaction mechanism of SN2. The nucleophile should also be in a sterically unhindered position. The strength of a nucleophile is greatly affected by its charge. With the increasing negative charge nucleophilicity also increases. For example OH- is a better nucleophile in comparison to water it can act as a very good nucleophile in the SN2 reaction.
Effect of solvent
The solvent will affect the rate of reaction in such a way that polar aprotic solvents are better solvents in comparison to polar protic solvents because polar protic solvents tend to form hydrogen bonds with the nucleophile thereby lowering its tendency to attack the carbon with the leaving group. Some of the best examples for solvents that can be used in SN2 reaction mechanism is dimethyl sulfoxide, dimethylformamide, acetone, DMSO, etc.
Effect of leaving group
The very beginning of the reaction depends on the breaking of the bond between the carbon and the leaving group so the strength of the bond between carbon and the leaving group has a greater impact on the rate of SN2 reaction. Halides are commonly used as a leaving group except for fluorine because fluorine forms a very strong bond with carbon due to its high electronegativity nature. Tosylate is also a very good example for leaving group but OH- and NH2- are not used as a leaving group because of its strong bond formation with the carbon.
Also Read:
SN2 Reaction Rate
The order of SN2 reaction is second as it depends on both the concentration of substrate and the nucleophile and is present in the rate-determining step. The rate of SN2 reactions can be written as,
r=k[RX][Nu-]
Where r is the rate, and k is the reaction constant. Therefore the reaction rate will depend on the concentration of nucleophiles and the substrate which is undergoing attack. This means that the increasing concentration of them will increase the rate of SN2 reaction. The figure below shows the formation of the transition state and the corresponding formation of the product.
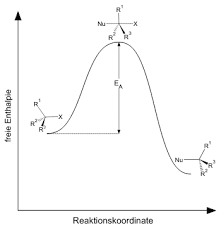
Energy profile diagram and the formation transition state.
NCERT Chemistry Notes:
SN1 and SN2 Reactions Examples
SN1 reaction is also a type of nucleophilic substitution reaction in which it is unimolecular that the order of this reaction is one. In comparison to SN2 reaction the reaction occurs in two steps. The first step involves the formation of a carbocation then the nucleophilic attack takes place resulting in the formation of the corresponding product. The stereochemistry of SN2 reaction is also different. The following reaction shows the SN1 and SN2 pathways.
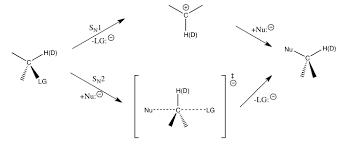
The formation of the transition state is only present in the case of SN2 reaction. And also there is no direct interaction between the given substrate and nucleophile; it only happens when the leaving group leaves. While in the case of SN2 reaction there is a direct collision between the nucleophile and the substrate. The solvent used in SN1 reaction is polar protic solvents. Water is the most commonly used solvent in SN1 reaction.
Also check-
