Sodium Nitrate (NaNo3) - Structure, Preparation, Properties, Uses, FAQs
Sodium nitrate is an alkali metal nitrate salt and the chemical formula of sodium nitrate is NaNO3. It is also known as Chile's saltpeter. Sodium nitrate is found exclusively in Chile and Peru. NO-3 The chemical name is nitrate. The compound consists of nitrate, NO-3, and the sodium cation that is Na+. Sodium nitrate is a white crystalline solid and has a high solubility in water. Sodium nitrate is also given the name white gold.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Sodium Nitrate Structure
- Preparation of Sodium Nitrate
- Properties of Sodium Nitrate
- Sodium Nitrate Uses
The mining of sodium nitrate from chile saltpeter was reduced due to the introduction of the Haber process that is ammonia is now produced in excess so it can be easily used for the production of sodium nitrate. Sodium nitrate is a very important compound since it contains nitrogen therefore it can be used as a fertilizer.
Sodium Nitrate Structure
As the sodium nitrate, a compound contains one sodium-ion that is Na+, and one nitrate ion that is NO-3, and the nitrate charge is -1. The nitrate ion has a trigonal planar geometry where the three oxygen atoms are bonded to the central atom of nitrogen one is doubly bonded and the two others are singly bonded to nitrogen. The overall formal charge on the nitrate and on is -1 and Na+ is coordinated to it as shown below. NaNO3 The chemical name is sodium nitrate.
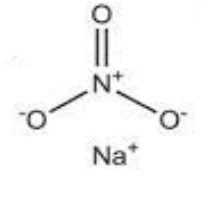
Structure of sodium nitrate
Preparation of Sodium Nitrate
Industrial sodium nitrate is prepared by a neutralization reaction between nitric acid and sodium carbonate. The following equation shows the preparation of sodium nitrate by these compounds.
NaHCO3+HNO3NaNO3+H2O+CO2
Another method for the preparation of sodium nitrate is by the reaction of sodium bicarbonate with nitric acid. The following equation shows the preparation of sodium nitrate by this method.
Na2CO3+2HNO3→2NaNO3+CO2+H2O
By the reaction of nitric acid with sodium hydroxide sodium nitrate is obtained. The following equation shows the preparation of sodium nitrate by this method.
NaOH+HNO3NaNO3+H2O
The reaction between sodium hydroxide and nitric acid is highly exothermic since sodium hydroxide is a strong base and also nitric acid is a strong acid. Show some other method is also adopted for the preparation of sodium nitrate which is by the reaction of sodium hydroxide with ammonium nitrate instead of the nitric acid. The following equation shows the preparation by this method.
NH4NO3+NaOH→NH4OH+NaNO3
| Related topics link, |
Properties of Sodium Nitrate
From the sodium nitrate formula NaNO3 we get that it is a compound formed by sodium and nitrate compounds. The preparation of sodium nitrate involves the use of strong acid and a strong base so the compound formed after the reaction will be a neutral salt. So sodium nitrate is a compound with a pH value of 7. Sodium nitrate is a white crystalline solid with a molecular mass of NaNO3 is 84.9 g per Mol and has a density of 2.257 grams per cubic centimeter. The boiling point of sodium nitrate is 38 degrees Celsius and the melting point is 308 degrees Celsius.
Sodium nitrate is a crystalline structure and has two crystalline structures possible, rhombohedral and trigonal. Sodium nitrate has a sweet odor and is highly soluble in water at a temperature of 25 degrees Celsius. This compound also has a high solubility with ammonia. While dissolving sodium nitrate in water it dissociates into sodium ions and nitrate ions that are Na+ and NO-3. Sodium nitrate is a very strong oxidizing agent.
Effect of Sodium Nitrate on Plant Growth
Nitrogen is a very important mineral for the growth of plants. But as many of the soils do not contain nitrogen naturally the application of nitrogen with any readily available nitrogenous fertilizers often makes the growth of plants effective. Sodium nitrate is the readily available nitrogen as fertilizer which increases crop growth and production.
The solubility of sodium nitrate in water is high so the application of sodium nitrate fertilizers over the soil should be well-adapted. How many methods are there in order to reduce the wastage of sodium nitrate or the leaching of sodium nitrate with the water? Larger quantities are applied to place them in the sand just because of their solubility with the water. Several methods are adopted by the farmers to reduce the loss of sodium nitrate with water like applying grilling before plating and the second and the latter application as a side dressing for top dressing etc.
Also Read:
Sodium Nitrate Uses
- As it is a salt containing nitrate so the content of nitrogen is high it is used as fertilizer. Sodium nitrate fertilizer is now widely used in the agricultural field.
- A hybrid form of an Aqua regia is prepared with the help of sodium nitrate that has the ability to dissolve gold.
- Sodium nitrate is widely used as a preservative or as a food additive. It is also used to stabilize the meat color texture improve and development of meat flavor, reduce microbial activities, etc. sodium nitrate is used.
- It is used as an oxidizer for several types of fireworks.
- This is one of the major components of instant cold packs.
- In some solar power plants, it is used for the storage and transfer of heat.
- Sodium nitrate is used for wastewater treatment by promoting the growth of Nitrosomonas bacteria.
- Sodium nitrate is used as a substitute for potassium nitrate which is used in gunpowder.
Decomposition of Sodium Nitrate
Most of the nitrates decompose upon heating and the thermal decomposition of sodium nitrate takes place at a temperature above 800 degrees Celsius. The decomposition of sodium nitrate by the application of heat can be given by the following chemical equation.
2NaNO3→ 2NaNO2+O2
From the NaNO3 decomposition oxygen is formed as a byproduct, as a colorless odorless gas after the reaction. The reagent sodium nitrate is changed to sodium nitrite.
Also Read:
Frequently Asked Questions (FAQs)
The NaNO3 chemical name is Sodium nitrate.
Sodium nitrate is formed by the reaction of a strong base and strong acid. And is a salt compound therefore it is neither acidic nor basic, a neutral compound with a pH value of 7.
Sodium nitrite and oxygen gas were obtained. That is,
2NaNO3∆→ 2NaNO2+O2
The charge of sodium is +1.
The no3 chemical name is nitrate ion.
The formula of sodium nitrate is NaNO3
Nitrates have a very wide application. It is a component of sodium nitrate which is itself used as fertilizer in the agricultural sector. The nitrate ions are responsible for the use of sodium nitrate as a fertilizer. Plants will utilize nitrates for making proteins for their healthy growth. That is the component nitrogen present in the nitrate is responsible for this. Plants will absorb nitrates through their roots from the water. The solution of sodium nitrate is a neutral compound.
-1
Sodium nitrate.
84.99 g/mol.
Sodium nitrate should be stored in a cool, dry place away from incompatible substances, such as strong acids or flammable materials. It should be kept in tightly closed containers to prevent contamination and moisture absorption. Proper safety measures should be followed, especially in industrial settings.
Sodium nitrate is generally recognized as safe when used in food preservation at regulated levels. However, excessive consumption can lead to potential health issues. It is converted in the body to nitrite, which can form nitrosamines, compounds that may be carcinogenic. It is advisable to consume processed meats containing sodium nitrate in moderation.
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM