Sodium Oxide (Na2O) - Oxide ion, Formation, Structure, Properties, FAQs
Sodium oxide as the name suggests is a chemical compound with chemical formula Na2O. It has many applications in glass and ceramics industries. Sodium oxide is an alkali metal oxide and it is also a basic anhydride form of sodium hydro oxide, and is denoted by the symbol NaOH. In presence of air, sodium oxide burns with a white light which is very vivid but in existence of oxygen it burns even more rapidly as compared with air. After burning it forms a white powder look a like substance which is called sodium oxide. The molecular mass of sodium oxide is 61.97g/mol. Sodium oxide is a chemical compound which is yellow in color and the nature of sodium oxide is crystalline solid. Sodium oxide has a melting point of 1132oC.
This Story also Contains
- Formation of sodium oxide
- Properties of sodium oxide
- Structure of sodium oxide
- Oxide ion
- Applications of sodium oxide
Formation of sodium oxide
Sodium oxide is formed with the help of the reaction of sodium hydroxide with sodium which is in metallic in form. Instead of metallic sodium, sodium nitrate or with sodium peroxide, we can also produce sodium oxide. In this reaction, hydrogen is produced as a by-product. The chemical equation is given below
NaOH+ NaO→Na2O+H2
By thermal decomposition of sodium carbonate at the temperature of 850oC, we can get sodium oxide along with by-product carbon dioxide.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Sodium oxide with water
Sodium oxide is highly insoluble in water. Sodium oxide reacts vigorously in presence of water, giving sodium hydro oxide as the product. Thus, sodium oxide should not be kept in presence of water, it should be stored away in clean and dry environment, because of its inflammable nature.
Properties of sodium oxide
In presence or when exposed to water, sodium oxide changes into sodium hydroxide. The reaction of sodium oxide with water is very vigorous because of large exothermic activities taking place. Thus, sodium oxide should be stored in a clean and dry place, away from water. Alkali oxides like M2O has applications in crystallization of the structure of antifluorite. Thus, the location of the anions and cations relatively gets backwards in comparison to the location of calcium difluoride. The cause of this process is due to ions of sodium that tetrahedrally coordinate with 4 oxides of ions and cubic oxides that co ordinate with Na ions which is 8 in number. Na is nothing but the chemical name of sodium metal. The full form of Na is sodium metal.
Structure of sodium oxide
The structure of sodium oxide comprises of molecules which are made up of one oxygen anion and two sodium cations. Therefore, sodium oxide has two sodium oxygen ionic bonds. The structure of sodium oxide is given below
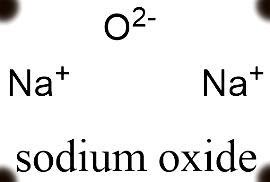
The Ph of Na2O is almost 14 when dissolved in aqueous medium, making it complete basic in nature.
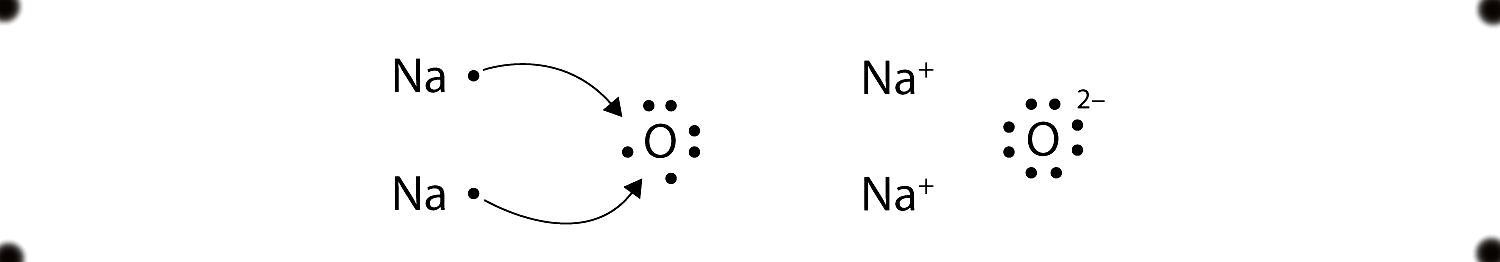
The Lewis structure of Na2O is given below
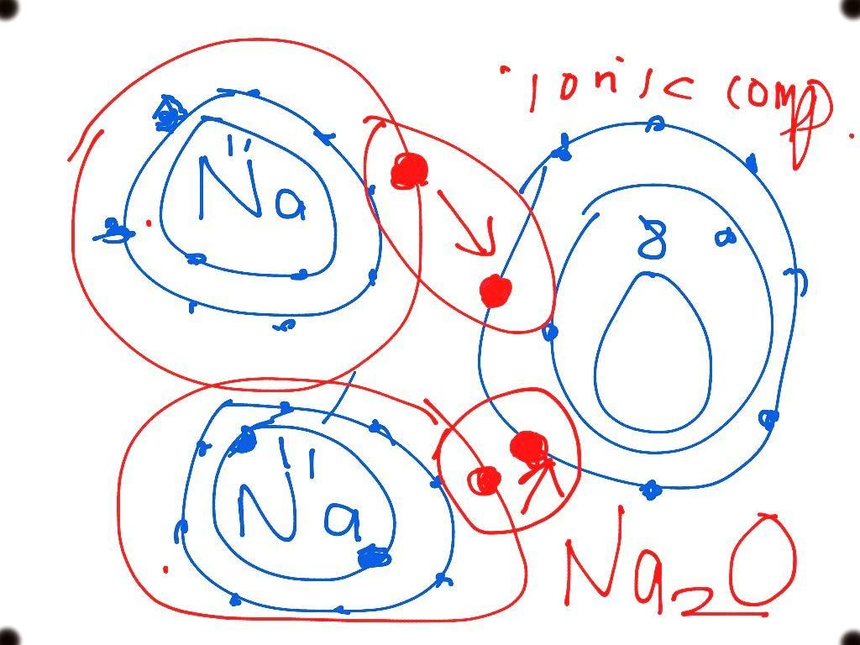
The ionic bonding diagram of sodium oxide is given below
Oxide ion
Oxide as the name suggests is the two anion of oxygen atom. Oxides of metal usually comprises of anion of oxygen atom in the oxidation state of -2. Metals are mostly oxidized in presence of oxygen in water or in the air. Even the so- called pure metals or metals which are considered of high grade often gets oxidize by developing a coating. With different concentration of oxygen being oxidized with the metal in presence, many metals can form multiple oxides. Examples of such metals are iron, carbon, nitrogen, silicone, titanium, and lithium. In the cases of these metals, the oxides are differentiated by specifying the number of atoms in the complex, as in carbon dioxide and carbon monoxide, or by giving priority to the oxidation number as in the case of iron oxides.
The formula of oxide ion is 02-.
| Related topics, |
Formation of oxide
Because of the electronegative nature of oxygen atom, it forms chemical bonds which are stable with all elements which give corresponding oxides. The most common tracks for corrosion or oxidation of metal are oxidation by oxygen and hydrolysis. The presence of addition of oxygen and water is most corrosive in nature. In the existence of oxygen and water, few elements namely sodium react vigorously to give hydroxides, thus alkali and alkaline earth metals are in the metallic form or native form, found in nature.
Cesium being the most reactive in nature has applications in vacuum tubes as a getter and aqueous medium of sodium and potassium are used in dehydration operations of organic solvents. The exterior of many metals comprises of hydroxides and oxides in the existence of air. In electrolytic anodizing, the oxide layer of Al can be constructed to thicker in concentration. Many metals in grandly grounded powder form are vigorously inflammable in presence of air. Oxides are differentiated into many types decided on the factors like nature and properties the compounds have, like acidic oxides, neutral oxides, basic oxides and amphoteric oxides etc.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 The S-Block Elements
- NCERT notes Class 12 Chemistry Chapter 10 The S-Block Elements
Applications of oxides
For practical experiments in laboratories, oxides are used for the formation of salts.
It has applications in production of slag.
Oxides has applications in the production of motor industries.
Oxides are also used as a drying agent.
Applications of sodium oxide
Due to its high solubility and thermal stability, sodium oxides have the best applications in ceramic, glass and optic manufacturing industries. Oxidized compounds are good conductors of electricity. Due to the solubility of sodium oxide, it is used in making clay apparatus, structural constituents of aeronautical industries which are light in weight, ceramic components and in electrical field. Due to the ability of sodium oxide of ionic conductance it has applications for manufacturing fuel cells.
Sodium oxides as the name suggest are anhydride in basic form which make it more suitable for reaction with acids. Sodium oxides are accessible in many forms like tablets, pellets, spluttering targets, pieces and non-powder. Sodium oxide is also used to make glazes, being an active flux to give color response. It is also used in structures as a primary deflocculant in casting slips. Sodium oxides are used in glass industries in cooperation of silicone and specific additives.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
In Chemistry, Na is the symbol for the Sodium. It is derived from the Latin word 'Natrium'.