Sodium Phosphate (Na3PO4) - Structure, Molecular Mass, FAQs
What is sodium phosphate?
Sodium phosphate is a salt formed when sodium (Na+) and phosphate (PO43−) ions combine. Sodium phosphate formula is Na3PO4. Sodium phosphate and its salt may exist in hydrated or anhydrous form. Commonly known as phospho soda, is saline purgative (purgative is a medicine for better digestion). Sodium phosphate or its other forms are produced when hydrogen atom(s) in phosphoric acid (H3PO4) are replaced by sodium atoms. Removal of one hydrogen atom forms monobasic form of sodium phosphate, removal of two hydrogen atoms forms dibasic form of sodium phosphate and removal of all hydrogen atoms forms tribasic form of sodium phosphate. Being a salt, sodium phosphate and its other forms are crystalline solids or white powders. They are colorless to white in appearance.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is sodium phosphate?
- Family of sodium phosphate
- Sodium phosphate uses
- Effects of sodium phosphate
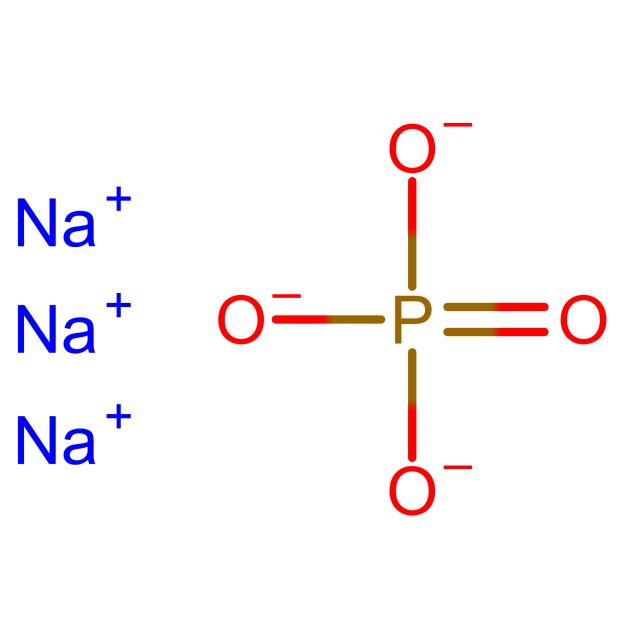
Figure 1 Na3PO4 structure.
Sodium phosphate is also known as sodium orthophosphate, tribasic sodium phosphate, trisodium phosphate. Density of trisodium phosphate is 1.62 g/cm3. Sodium phosphate molecular weight (or molar mass of sodium phosphate) is 163.94 g/mol.
Boiling point of sodium phosphate is 100 °C and the melting point of sodium phosphate is 1,583 °C. The value of trisodium phosphate solubility is ‘soluble in water’.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Family of sodium phosphate
Monophosphates
Sodium monophosphate salts are mostly derived from orthophosphate (PO43−), dihydrogen phosphate (H2PO4−) and hydrogen phosphate (HPO42−).
Some name and the formula of different sodium phosphate is given below.
monosodium phosphate (anhydrous) NaH2PO4 ,disodium phosphate (anhydrous)Na2HPO4, disodium phosphate (dihydrate) HNa2PO4(H2O)2, disodium phosphate (heptahydrate)HNa2PO4(H2O)7, monosodium phosphate (monohydrate) NaH2PO4(H2O), monosodium phosphate (dihydrate) NaH2PO4(H2O)2, disodium phosphate (octahydrate) HNa2PO4(H2O)8, disodium phosphate (dodecahydrate) HNa2PO4(H2O)12,
Diphosphates and polyphosphates
A number of important salts are produced when sodium reacts with pyrophosphates; also known as Diphosphates, triphosphates and high polymers.
Some name and formula are given below.
monosodium diphosphate (anhydrous) NaH3P2O7, disodium diphosphate (hexahydrate) Na2H2P2O7(H2O)6, trisodium diphosphate (anhydrous) Na3HP2O7, trisodium diphosphate (monohydrate) Na3HP2O7(H2O), disodium diphosphate (anhydrous) Na2H2P2O7, trisodium diphosphate (nonahydrate) Na3HP2O7(H2O)9, tetrasodium diphosphate (decahydrate) Na4P2O7(H2O)10
Triphosphate salts of sodium like tetraphosphates and sodium triphosphate are also known. Metaphosphates, a name given to cyclic phosphates such as Na3P3O9 trimer sodium trimetaphosphate and tetramer Na4P4O12 also exist.
When NaH2PO4 and Na2HPO4 are heated, polymeric sodium phosphates are formed. To form a specific polyphosphate annealing and heating process is altered. Some of such specific polyphosphate is Graham’s salt, Kurrol’s salt and Maddrell’s salt. Graham salt has a formula NaO(NaPO3)Na2. Kurrol’s salt and Maddrell’s salt has a general formula [NaPO3]n[NaPO3(OH)]2 where n can have a value upto 2000. . Kurrol’s salt and Maddrell’s salt are crystalline polyphosphates with a high molecular weight.
| Related topics link, |
Sodium phosphate uses
Sodium phosphate and its other forms are very commonly used in a lot of everyday products. This is because sodium phosphate is inexpensive and is nontoxic when taken in adequate amounts. Some sodium phosphate uses includes:
- Sodium phosphate is used in food and water treatment.
- Sodium phosphate is used as an emulsifier in processed cheese production.
- Sodium phosphate is used as a thickening agent in soups, etc.
- Sodium phosphate is used as a leavening agent for baked food.
- Sodium phosphate is used to control pH of processed food.
- Sodium phosphate is used as medicine for constipation.
- Sodium phosphate is used as pharmaceuticals to prepare the bowel for further medical processes.
- Sodium phosphate is used in detergents for the purpose of softening hard water
- Sodium phosphate is used in anti-rust solutions as an efficient solution.
- Sodium phosphate is used in fertilizers
- Sodium phosphate is used in animal food as preservative
- Sodium phosphate is used in soaps.
- Sodium phosphate is used as a food additive.
- Sodium phosphate particularly disodium pyrophosphate is often used as a buffer.
- Sodium phosphate is used in toothpaste to remove magnesium from saliva which prevents cavities and plaques.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 The S-Block Elements
- NCERT notes Class 12 Chemistry Chapter 10 The S-Block Elements
Effects of sodium phosphate
As discussed, sodium phosphate is used in bowel preparation for medical procedures like colonoscopy. High levels of sodium phosphate intake carries a hazardous risk of kidney failure. Sodium phosphates also play a major role in eutrophication of water. Eutrophication involves increased algal growth known as algal bloom due to presence of excess phosphate which in turn causes decreased oxygen levels in water. Sodium phosphates generally reach water bodies like ponds, rivers or lakes by washing off detergents, soaps, factories or fertilizers.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Sodium phosphate is a salt formed when sodium (Na+) and phosphate (PO43−) ions combine. Sodium phosphate and its salt may exist in hydrated or anhydrous form. Commonly known as phospho soda, is saline purgative (purgative is a medicine for better digestion). Sodium phosphate or its other forms are produced when hydrogen atom(s) in phosphoric acid (H3PO4) are replaced by sodium atoms. Removal of one hydrogen atom forms monobasic form of sodium phosphate, removal of two hydrogen atoms forms dibasic form of sodium phosphate and removal of all hydrogen atoms forms tribasic form of sodium phosphate. Being a salt, sodium phosphate and its other forms are crystalline solids or white powders. They are colorless to white in appearance. Sodium phosphate chemical formula is Na3PO4.
Na2HPO4 name is Disodium phosphate and Na3PO4 compound name is trisodium phosphate.
Na2PO4, trisodium phosphate is slightly acidic in nature based on its pKa value.
Sodium phosphate molecular weight (or molar mass of sodium phosphate) is 163.94 g/mol.
Na3PO4 is phosphate salt of sodium.
Na2HPO4, monosodium phosphate is neutral that is neither acid nor base.
Sodium phosphide formula is Na3P.
Molecular weight of phosphate is 94.971361 g/mol. It can be calculated by adding molecular weight of phosphorus (molecular weight of one P atom is 30.973761 g/mol) and molecular weight of four oxygen atoms (molar mass of one oxygen is 15.9994 g/mol).
Sodium phosphate enema bp uses are:
Sodium phosphate enema is used to treat constipation.
Sodium phosphate enema is used to clean the gastrointestinal tract usually before colonoscopy.
sodium phosphate monobasic molecular weight is 119.98 g/mol.
As discussed, sodium phosphate is used in bowel preparation for medical procedures like colonoscopy. High levels of sodium phosphate intake carries a hazardous risk of kidney failure. Sodium phosphates also play a major role in eutrophication of water. Eutrophication involves increased algal growth known as algal bloom due to presence of excess phosphate which in turn causes decreased oxygen levels in water. Sodium phosphates generally reach water bodies like ponds, rivers or lakes by washing off detergents, soaps, factories or fertilizers.
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM

