Sodium Thiosulfate - Structure, Application, Preparation, Uses, FAQs
The inorganic compound with the chemical formula Na2S2O3.XH2O is called sodium thiosulfate. XH2O suggested that it is present in its hydrated form. Generally it is present in its a pentahydrate a form that is the chemical formula is Na2S2O3.5H2O. It is a white colorless compound and it loses its water easily and dissolves well in water.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Na2S2O3 structure
- Uses of sodium thiosulphate
- Chemical reactions of sodium thiosulphate
- Preparation of sodium thiosulphate
- Hypo solution
The molecular mass of sodium thiosulfate is 158.11 gram per mole when it is present in the anhydrous former and if it is in the hydrated form that is pending hydrated form the value of molecular weight is 248.18 gram per mole. It is a colorless crystal and the density of Na2S2O3 is 1.667 grams per centimeter cube. The melting point of sodium thiosulfate pentahydrate is 321.4k and its boiling point is 100 degrees Celsius.
The solubility in water is high but it is negligible soluble in alcohol. Sodium thiosulfate is prepared by heating sodium sulfite solution with sulfur or by heating sodium hydroxide solution with sulfur. The following equation represents the formation of sodium thiosulphate.
6NaOH+4S→Na2S2O3+2Na2S+3H2O
Sodium thiosulphate has also possessed some other names like sodium hyposulphite, and hyposulphite of sodium, hypo, etc.

Sodium thiosulfate crystals
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Sodium thiosulfate has many applications; it is used extensively in gold mining, for the water treatment, for the development of a silver base as a photographic film and prints. One of the important applications of sodium thiosulphate is it can be used as a medicine for cyanide poisoning. In addition to that there are some other medical uses also it is used for the treatment of ringworm and tinea versicolor and also for treating the side effects caused during the chemotherapy treatment.
Thiosulphate has a wide application in the medical field just because it is a non-toxic material. But when sodium thiosulfate is decomposed it will produce sulfur dioxide fumes that are toxic and can cause irritation to skin eyes and even mucous membranes. Thiosulfate is used in analytical chemistry that is in the iodometric titration. Thiosulfate anion reacts with iodine to form iodide the following is the equation for the iodometric titration.
S2O32-+I2→S4O62-+2I-
In addition to the efficiency in this reaction with iodine thiosulfate also has an excellent shelf life so it is used as a titrant in iodometric titration.
Related Topics, |
Na2S2O3 structure
From the chemical formula of sulfur we get to know that it contains two sodium cations and a thiosulphate anion i.e. S2O32-. The central atom of sulfur forms one bond with the sulfur atom and the other bonded to the remaining oxygen atoms. The central sulfur atom forms a single and double bond in a resonating way. And so the stability of sodium thiosulphate is high. The following figure shows the sodium thiosulfate structure.
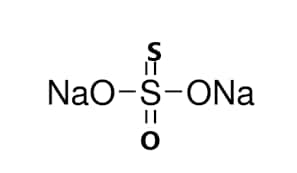
Structure of sodium thiosulfate
As shown in the structure, it forms a double bond with one oxygen and a sulfur atom.
Uses of sodium thiosulphate
Due to its low toxicity and highly stable structure, it has a wide range of applications.
Photography
Na2S2O3.5H2O is used in photography for the reduction of silver to metallic silver. That is for dissolving silver salt from the negatives sodium thiosulfate is used as a fixing agent.
Industrial application
It is used for the extraction of gold from its ore. And also it is used as a dechlorinating agent for small water bodies like aquariums, ponds, etc. It also has applications in the leather industry that is for the tanning process.
Medical application
For the preparation of pharmaceuticals, sodium thiosulfate is used. The most important application of sodium thiosulfate is used as an antidote for cyanide poisoning. And also it is used for reducing the side effects of chemotherapy.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 The S-Block Elements
- NCERT notes Class 12 Chemistry Chapter 10 The S-Block Elements
Chemical reactions of sodium thiosulphate
The solubility of thiosulfate in water is high and it produces sodium ions and thiosulfate ions. But the compound is insoluble in alcohol.
Na2S2O3+H2O→Na++S2O3-
The compound is a highly stable compound that we get from the structure itself but when it reacts with dilute acids it is decomposed to produce sulfur. The following equation shows the reaction of sodium thiosulphate with dilute hydrogen chloride acid.
Na2S2O3+2HCl→2NaCl+S+H2O +SO2
Although it is a stable compound this decomposes when external heat is supplied to it. The following reaction shows the decomposition of sodium thiosulphate by the application of heat.
4Na2S2O3→3Na2SO4+Na2S5
Preparation of sodium thiosulphate
Sodium thiosulfate, Na2S2O3 can be prepared by using two methods, one by heating sulfur with sodium sulfite solution. The other method is by boiling sulfur with sodium hydroxide solution. It is generally found in its pentahydrate form and so it is also known as sodium hyposulfite. Sodium thiosulphate is a good reducing agent and so it is used in many reactions.
For iodometric titration sodium thiosulfate is the titrant with the starch as an indicator since starch contains high amounts of iodine. During iodometric titration before iodine forms, a complex with starch sodium thiosulfate reduces iodine to iodide. So it is a very important inorganic salt with very high industrial applications.
Hypo solution
The hypo solution formula is Na2S2O3 and it is also known by the name of sodium thiosulphate solution. Hypo is present in liquid form and the color of the hypo solution is yellowish. Hypo solution is used for the photographic application i.e it is used as a photographic fixer for the development of the image. The hypo solution is used as the dechlorinating agent and also it is used in the iodometric titration as a titrant. It is the compound used for the treatment of cyanide poisoning. Hypochemical name is sodium thiosulfate.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
127u.
158.11g/mol.
Na2S2O3
Na2S2O3
Hypo solution is commonly known by the name of sodium thiosulphate. It is the solution form of sodium thiosulfate that is present in the liquid state. It is a basic compound with many applications in many fields. The chemical formula of hypo solution is similar to sodium thiosulfate that is Na2S2O3. It is used as an intravenous medication for the treatment of poisoning of metals, mainly cyanides. And also used for the treatment of diseases like Calciphylaxis, dermal lesions, etc. It is also used as an anti-calcification and inflammatory agent. It is used as a dechlorinating agent for water bodies like swimming pools, water bodies, etc., and is because of its low toxicity. The name hypo always represents sodium thiosulfate as there are some other compounds like sodium hypochlorite with a similar sound.
248
Na2S2O3
It is compound with many industrial as well as medical applications. Below are some of the important uses of sodium thiosulfate. It is used for the treatment of several metals poisoning mainly as an antidote in cyanide poisoning by converting cyanide to a soluble form and thereby removed through the urine.
In the mining of gold sodium thiosulfate is an important component.
It is used as an antifungal medicine on the skin to treat the infections caused by fungus.
For the process of tanning in the leather industry, sodium thiosulfate is used.
As a dechlorinating agent for water bodies like swimming pools, ponds, etc. sodium thiosulfate is used.
Na2S2O3
Sodium thiosulfate.
Also Read
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM
26 Sep'24 05:53 PM