Solubility and Henry's Law: Definition, Formula, Questions and Examples
The amount of gas that dissolves in a liquid at a given constant temperature is said to be directly proportional to the partial pressure of that given gas that comes in contact with the liquid. If the pressure is decreased, then the gas molecules leave the liquid and solubility will decrease.
This Story also Contains
- Henry’s Law
- Applications of Henry’s law
- Some Solved Examples
- Conclusion
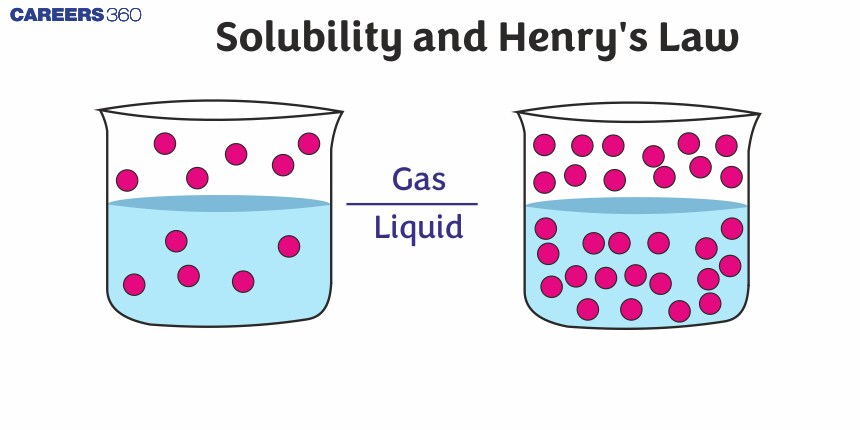
Henry’s Law
Henry was the first to give a quantitative relation between the pressure and solubility of a gas in a solvent. The law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution. If the solubility of gas is expressed in terms of its mole fraction in the solution, then it can be said that the mole fraction of gas in the solution is proportional to the partial pressure of the gas over the solution. Alternatively, the most commonly used form of Henry’s law states that “the partial pressure of the gas in vapor phase (P) is proportional to the mole fraction of the gas (x) in the solution” and is expressed as:
P=KHx
Here KH is the Henry’s law constant and has the same units as the units of pressure used in the equation. It can be seen that the plot between the partial pressure of the gas versus the mole fraction of the gas in solution will be a straight-line plot as shown in the figure given below.

Factors governing the value of KH
Different gases have different KH values at the same temperature. Also, the same gas has different values of KH at different temperatures which is shown in the table given below. The factors governing the value of Henry’s constant are given below
Nature of gas-solvent interaction
As gas-solvent interactions become stronger, the solubility will increase keeping the pressure constant and thus the value of KH will decrease. For example, HCl has a lower value of Henry’s constant as compared to O2
Temperature:
As the temperature increases, the solubility of the gas decreases, and hence keeping the pressure constant for the same gas, the value of KH will increase.
Gas | Temperature(K) | KH/kbar |
|---|---|---|
Helium | 293 | 144.97 |
Hydrogen | 293 | 69.16 |
Nitrogen | 293 | 76.48 |
Nitrogen | 303 | 88.84 |
Oxygen | 293 | 34.86 |
Oxygen | 303 | 46.82 |
Argon | 298 | 40.3 |
Carbon dioxide | 298 | 1.67 |
Formaldehyde | 298 | 1.83x105 |
Methane | 298 | 0.413 |
Vinyl chloride | 298 | 0.611 |
Most gases obey Henry’s law provided they are not highly soluble in the solvent and do not chemically react with it.
Applications of Henry’s law
(1) Soda bottle fizzes when opened: When the soda bottle is opened, then the pressure decreases in the bottle. Now, due to this decrease in pressure, the solubility of gas decreases. Now if we leave this bottle open for some time, then all fizz goes out and we do not feel the drinking.
(2) Anoxia at higher altitudes: This is the condition of tiredness and mental confusion at higher altitudes. At higher altitudes, pressure decreases and thus the solubility of oxygen gas in the body decreases which causes anoxia.
(3) Avoiding Bends in Scuba divers: When scuba divers go deep into the ocean, then pressure increases. Now due to this increase in pressure, the solubility of gases in the blood increases. Further, when these divers come up at the sea level, then pressure decreases, and the solubility of gases in the blood decreases. Due to this decrease in solubility, the gases come out of the capillaries in the form of bubbles which causes a serious medical condition called bends. To avoid bends, Helium is used in the diving tanks
Recommended topic video on (Solubility and Henry Law)
Some Solved Examples
Example 1
Question: From the given KH value, which of the following gases has the highest solubility in water?
Gas KH Value in K-bar
1)CO2: 1.67
2)N2: 80
3)Formaldehyde: 1.83 times 10-5
4) CH4: 0.413
Solution: Gases with higher KH values have lower solubility. Among the options, Formaldehyde has the lowest KH value, indicating it has the highest solubility in water. Therefore, the correct answer is Formaldehyde.
Example 2
Question: The solubility product of Cr(OH)3 at 298 K is 6.0 times 10-31 . What is the concentration of hydroxide ions in a saturated solution of Cr(OH)3 ?
1) $(18 \times 10^{-31})^{1/2}$
2) $(2.22 \times 10^{-31})^{1/4}$
3) $(18 \times 10^{-31})^{1/4}$
4) $(4.86 \times 10^{-29})^{1/4}$
Solution: The dissociation of $Cr(OH)_{3}$ can be represented as $Cr(OH)_{3}\rightarrow Cr^{+3} + 3OH^{-}$ . The solubility product is given by $K_{sp} = s(3s)^{3} = 27s^{4}$ . Setting this equal to $6.0 \times 10^{-31}$ and solving for s gives $s = (\frac{6.0}{27} \times 10^{-31})^{1/4}$ . The concentration of hydroxide ions is $[OH^{-}] = 3s = 3 \times (\frac{6.0}{27} \times 10^{-31})^{1/4} = (18 \times 10^{-31})^{1/4}$ . Thus, the correct answer is option 3.
Example 3
Question: Which one of the following statements regarding Henry's law is not correct?
1) The higher the value of $K_{H}$ at a given pressure, the higher the solubility of the gas in the liquids.
2) Different gases have different $K_{H}$ values at the same temperature.
3) The mole fraction of the gas in the solution in the vapor phase is proportional to the partial pressure of the gas.
4) The value of $K_{H}$ increases with the increase in temperature.
Solution: The incorrect statement is option 1. According to Henry's law, a higher $K_{H}$ value indicates lower solubility of the gas in the liquid. Therefore, the correct answer is option 1.
Example 4
Question: For the solution of the gases w, x, y, and z in water at 298 K, Henry's law constants $( K_{H} )$ are 0.5, 2, 35, and 40 kbar, respectively. Which plot correctly represents the given data?
1) Correct
2) Incorrect
3) Incorrect
4) Incorrect
Solution: The correct plot would show a negative slope since $K_{H}$ values are inversely related to solubility. The gas with the highest $K_{H}$ will have the lowest solubility, leading to a downward trend. Therefore, the correct answer is option 1.
Example 5
Question: On increasing the temperature, the value of $K_{H}$ will:
1) Increase
2) Decrease
3) Remain the same
4) Either increase or decrease
Solution: As the temperature increases, the solubility of gases generally decreases, leading to an increase in the value of $K_{H}$. Thus, the correct answer is option 1.
Conclusion
Henry's law is one of the basic propositions for the relationship of gas solubility with partial pressure and has very important implications in several areas. From carbonated soft drinks and concerns for scuba diver safety to the health of aquatic ecosystems and uses in industry, this law underlines much of both our daily lives and scientific research. The finer the understanding of the principles behind Henry's Law and its applications, the more undeniable the need for the natural world and viable environments.
