States of Matter - Notes, Topics, Books. FAQs
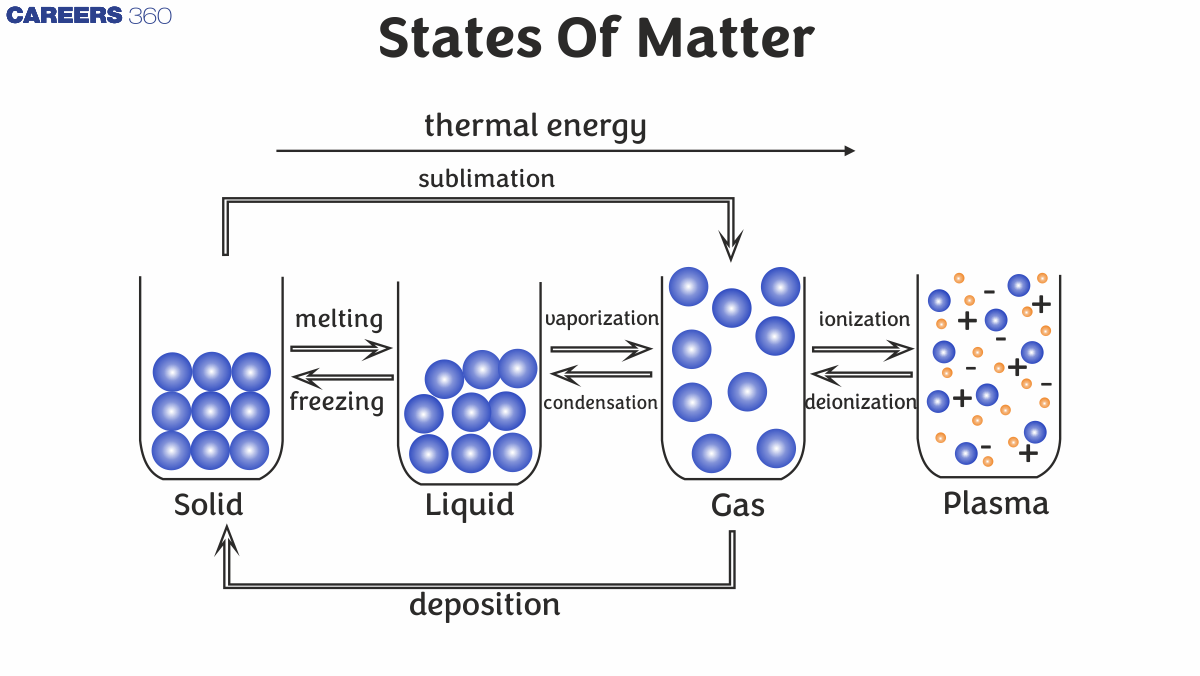
In the universe, matter exists in three different forms, i.e solid, liquid and gas. The characteristic properties of materials that we can see are of the bulk but not of the single particle. Physical properties of the matter may change in different physical forms but chemical properties do not change. For example water, it can exist in three different forms, i.e ice, water and gas. In all of its three forms, its physical properties are different from each other but chemical properties remain the same. There are other states of matter exist as well but those are beyond the scope of this chapter.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
Important Topics of States of Matter
Intermolecular Forces vs Thermal Interactions
Intermolecular forces are the attractive or repulsive forces between molecules, such as hydrogen bonding or Van der Waals forces. Thermal interactions involve the kinetic energy of molecules due to temperature. The balance of these determines a substance's state. Stronger intermolecular forces favour solids or liquids, while high thermal energy favours the gaseous state.
Gas
The gas is defined as the state of matter in which particles move freely, with negligible intermolecular forces and high kinetic energy. Gases have no fixed shape or volume, expand to fill their container, and are highly compressible.
Boyle’s Law
Boyle’s Law states that the volume of a gas is inversely proportional to its pressure at constant temperature (P∝1/V). This relationship explains gas compression and expansion during constant-temperature processes.
Charles’ Law
Charles’ Law in gaseous states held that the volume of a gas increases linearly with temperature at constant pressure (V∝T). This concept is important for understanding the thermal expansion of gases.
Gay-Lussac’s Law
Gay-Lussac’s Law held that the pressure of a gas is directly proportional to its absolute temperature at constant volume (P∝T). This relationship highlights the role of temperature in pressure changes.
Avogadro’s Law
Another most important law is Avogadro’s Law. It states that the equal volumes of gases at the same temperature and pressure contain the same number of molecules. This principle underpins the concept of molar volume and the ideal gas law.
Dalton’s Law of Partial Pressure
As per the Dalton’s Law of partial pressure, the total pressure of a gas mixture equals the sum of the partial pressures of its components. Dalton’s Law of partial pressure is important for studying gas mixtures and applications like atmospheric science.
Derivation of Ideal Gas Equation
The ideal gas equation combines Boyle’s, Charles’, and Avogadro’s laws to describe the relationship among pressure, volume, temperature, and the number of moles of a gas under ideal conditions. The equation which is obtained after the Derivation of ideal gas equation is PV=nRT.
Graham’s Law: Diffusion and Effusion
Graham’s Law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. Graham's Law helps in understanding how lighter gases diffuse or effuse faster than heavier gases.
Kinetic Theory of Gases
This theory describes the motion of gas particles, linking macroscopic properties like pressure and temperature to microscopic particle behaviour. Kinetic Theory of gases assumes elastic collisions and no intermolecular forces.
The Deviation of Real Gas from Ideal Gas Behaviour
Real gases deviate from ideal gas behaviour due to intermolecular forces and the finite size of gas particles, especially under high pressure or low temperature. The deviation of real gas from ideal gas behaviour is understood mathematically by Van der Waals equation.
Liquefaction of Gases
Liquefaction of gases involves converting a gas into a liquid by lowering temperature or increasing pressure. This process depends on the gas's critical temperature and pressure which is further explained by Van der Waals forces.
Liquid State
Liquid is the state of matter where volume is fixed but no fixed shape, with particles in close contact that flow due to moderate intermolecular forces. Liquids exhibit properties like surface tension, viscosity, and capillarity.
Crystalline and Amorphous Solids
Crystalline solids have an ordered arrangement of particles, while amorphous solids lack long-range order. Crystalline and amorphous solids differ in various properties such as melting points and mechanical strength.
Crystal Lattices and Unit Cells
Crystal lattices are three-dimensional arrangements of particles, and unit cells are the smallest repeating units that define the lattice structure. Crystal lattices and unit cells are fundamental concepts to solid-state chemistry.
Close Packing in Solids in Three Dimensions
Close packing in solids maximizes particle density. Hexagonal close packing (HCP) and cubic close packing (CCP) are common arrangements where the packing efficiency is highest.
Packing Efficiency of a Unit Cell
Packing efficiency of a unit cell is the percentage of space occupied by particles in a unit cell. Packing efficiency is highest in close-packed structures like FCC (74%) and lowest in simple cubic structures (52%).
Structures of Ionic Solids
Ionic solids are solids having alternate arrangement of positive and negative ions in lattice. Structure of ionic solids depends on factors like ionic size and charge which determines properties like hardness and conductivity.
Defects in Solids
Solids can have defects like vacancies, interstitials, or substitutional impurities. Defects in solids influence properties such as electrical conductivity, strength, and optical behaviour.
Electrical Properties of Solids
Solids can be conductors, semiconductors, or insulators, based on their band structure. Electrical properties of solids depends on electron mobility and the availability of conduction bands.
Magnetic Properties of Solids
Solids have magnetic properties like diamagnetism, paramagnetism and ferromagnetism. Magnetic properties of solids depends upon electron spin and magnetic domain alignment. These properties have applications in various electronic appliances.
Bragg’s Law
Bragg’s Law (nλ=2dsinθ) explains X-ray diffraction in crystals. It is related to the angle of incidence to lattice spacing. It is essential for determining crystal structures.
| Also read, |
Overview of the States of Matter
In the universe, matter exists in three different forms, i.e, solid, liquid and gas. All these three forms are equally important in their own forms such as solids like chair we use for sitting and the car we use for travel. Liquids like water are important for drinking and gases like oxygen are important for breathing, etc.
The table given below explains the major differences between the solids, liquids and gases.
Property | Solid | Liquid | Gas |
Tightness | Very tightly packed | Tightly packed | Loosely packed |
Intermolecular space | Minimum | Intermediate | Maximum |
Force of attraction | Maximum | Intermediate | Minimum |
Kinetic Energy | Minimum | Intermediate | Maximum |
Density | Maximum | Intermediate | Minimum |
Volume | Fixed | Fixed | Variable |
Shape | Fixed | Variable | Variable |
Compressibility factor | Minimum | Intermediate | Maximum |
Gas Laws
For explaining the phenomena of the gases, researchers over the years have developed various laws i.e, Boyle's law, Charle's law, Avogadro's law, etc.
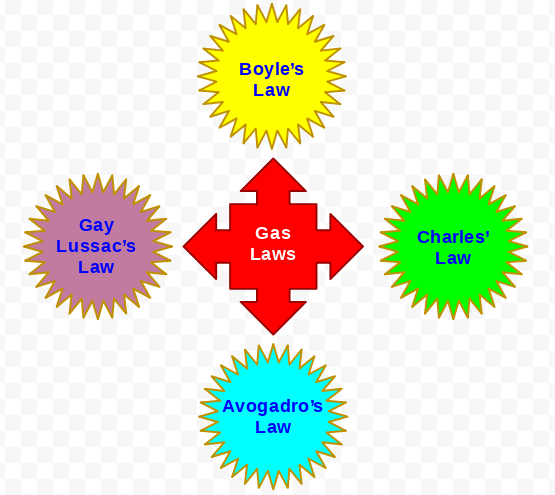
Important laws for gases
(i) Boyle's Law: This law states that at constant temperature and amount of gas, the pressure of a gas is inversely proportional to its volume. Mathematically, this can be represented as follows: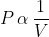
 Where k is proportionality constant.This law can also be represented as follows:
Where k is proportionality constant.This law can also be represented as follows: This law can also be expressed graphically. In the graph given below, the pressure of the gas is being measured at different temperatures.
This law can also be expressed graphically. In the graph given below, the pressure of the gas is being measured at different temperatures.
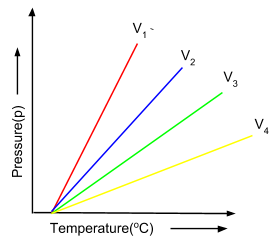
(ii) Charles' Law: This law states that for every 10 rise in temperature for fixed amount of gas, the volume of a gas increases by 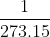 of the original volume of the gas at 00C. Mathematically, this law can be represented as follows:
of the original volume of the gas at 00C. Mathematically, this law can be represented as follows: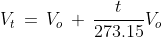
The graph below shown is of the different isobars, which are depicting the increase of volume on increasing the temperature.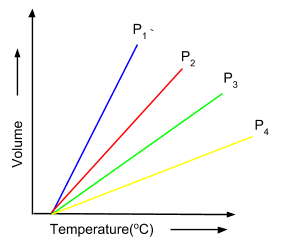
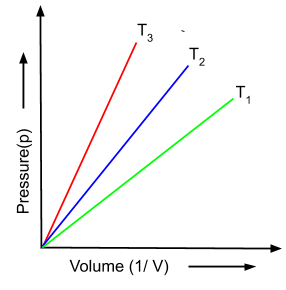
(iii) Gay Lussac's Law: This law states that for a fixed amount gas at constant volume, the pressure is directly proportional to the temperature.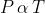
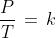 where k is proportionality constant. This law can be understood from the graph given below as well. In this graph, the pressure is increasing with the increase in temperature at different volumes of gas.
where k is proportionality constant. This law can be understood from the graph given below as well. In this graph, the pressure is increasing with the increase in temperature at different volumes of gas.
(iv) Avogadro's Law: This law states that equal volume of all gases at the same temperature and pressure contain equal number of molecules
Ideal Gas Equation
The three given laws when combined together in a single equation then that is known as ideal gas equation.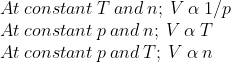 Thus,
Thus,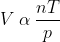 ;
;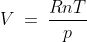
Therefore,
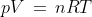 ; R = Universal gas constant and has value 8.314Jk-1mol-1s
; R = Universal gas constant and has value 8.314Jk-1mol-1s
(vi) Dalton's Law of Partial Pressures: This law states that the pressure exerted by the non-reactive gases is equal to the pressure exerted by the individual gases. In this case, the pressure exerted by the individual gases is called partial pressure.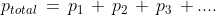 Here p1, p2, p3... are the partial pressures of individual gases
Here p1, p2, p3... are the partial pressures of individual gases
Kinetic Theory of Gases
This theory was proposed to understand the behaviour of gases in a better way. This is known as 'kinetic theory of gases'. The main postulates of this theory are as follows:
- The gas is comprised of a large number of tiny particles known as molecules. These molecules are completely identical in size, shape and mass.
- The volume of a single molecule is very small as compared to the total volume of a gas.
- There is no force of attraction and repulsion between these gaseous molecules.
- The molecules of these gases are always in constant and random motion.
- The molecules of the gas always move in random in directions in straight lines. During this motion, these particles collide with each other and with the walls of the container.
- Collisions of the molecules are perfectly elastic and thus their energy is always conserved before and after the collision.
- At any instant of time, these molecules move with different speed and thus these molecules have different kinetic energies.
Behaviour of Real Gases
All the laws that we have studied so far are applicable to the gases if we assume that these gases are ideal gases. But in reality these gases behave quite differently and thus these gases are known as the 'real gases'. In the figure below, the deviation of the behaviour of gases is shown.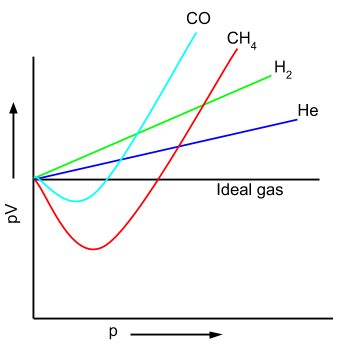
To analyse the real gases behaviour, pV vs p curve is plotted. In this curve, helium and hydrogen gases are showing positive deviation from an ideal gas. Other two gases like carbon monoxide and methane are showing negative deviation to a certain point after that these gases cross the ideal gas and show the positive deviation. This deviation of the behaviour of gases from ideal gas is mainly because of two factors:
- There is a force of attraction between the gas molecules exist. Thus the gases liquify at high pressure and low temperature.
- Volume of the gas molecules is not negligible as compared to the space containing it.
On the basis of these observations, a new gas equation was given also known as van der Waals equation. Mathematically, it can be written as below: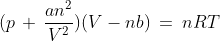 here, n is the number of moles of gas and a and b are van der Waals constants and their value depend on the properties of the gas.
here, n is the number of moles of gas and a and b are van der Waals constants and their value depend on the properties of the gas.
The deviation of gases from ideal behaviour is expressed in terms of the compressibility factor(Z), which is represented as follows: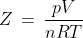
- For ideal gases, Z=1.
- For real gases, either Z>1 at high pressures or Z<1 at intermediate pressures.
Liquefaction of gases
There are three main terminologies that we used to describe the liquefaction of gases.
Critical Temperature: It is the temperature above which any gas does not remain liquid. For carbon dioxide, this temperature is
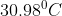 .
.- Critical Pressure: It is the pressure at any particular temperature at which a gas starts to liquify. At pressures higher than this value, the gas remains as a liquid. For carbon dioxide, this value is 73 atmospheric pressure at
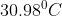 .
. - Critical Volume: It is the volume of one mole of a gas at critical temperature.
Liquid State: Liquid is one of the three forms of matter. In liquids, intermolecular forces are stronger than gases but weaker than solids. Thus these have a tendency to flow and occupy the shape of the container. There are few important properties that you need to consider such as vapour pressure, surface tension, and viscosity.
How to prepare for States of matter?
This chapter is a part of Physical chemistry. This chapter is one of the most important chapters of the complete chemistry syllabus. Its concepts, laws, numericals and graphs all are important both for basic foundation of chemistry and for scoring good marks in the examination.
Before reading this chapter, first, you must have the basic knowledge of the mole concept.
You must observe why gases are not ideal in real and on what conditions these gases become ideal. Learn carefully about the graphs as some questions will be direct from graphs.
Overall this chapter is very simple, just be regular and be consistent in your numerical practice.
Prescribed Books for the States of Matter
First, you must finish the class XI NCERT textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and O.P. Tandon. You must definitely solve the previous year papers. Meanwhile, in the preparation, you must continuously write the mock tests for the depth of knowledge. Our platform will help you to provide with the variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Also read,
Frequently Asked Questions (FAQs)
The key topics covered in the states if matter are :
- Differences between solid, liquid, and gas
- Gas laws such as Boyle's, Charles', and Gay-Lussac's laws
- Avogadro's law
- The ideal gas equation
- Kinetic theory of gases
- Real gas behaviour
- Liquefaction of gases
- Properties of liquids and solids, including crystal structures and defects.
This chapter forms the foundation for understanding physical and chemical processes, including gas laws, molecular behaviour, and solid-state properties. It is frequently tested in competitive exams like JEE, NEET, and other entrance tests.
Real gases deviate due to intermolecular forces and finite molecular volumes, especially at high pressures and low temperatures. The Van der Waals equation modifies the ideal gas law to account for these factors.
Intermolecular forces determine the strength of attraction between particles. Strong intermolecular forces favour solids, moderate forces favour liquids, and weak forces lead to the gaseous state. These forces influence properties like boiling point, melting point, and compressibility.
The key topics covered in the states if matter are :
- Differences between solid, liquid, and gas
- Gas laws such as Boyle's, Charles', and Gay-Lussac's laws
- Avogadro's law
- The ideal gas equation
- Kinetic theory of gases
- Real gas behaviour
- Liquefaction of gases
- Properties of liquids and solids, including crystal structures and defects.
This chapter forms the foundation for understanding physical and chemical processes, including gas laws, molecular behaviour, and solid-state properties. It is frequently tested in competitive exams like JEE, NEET, and other entrance tests.
Real gases deviate due to intermolecular forces and finite molecular volumes, especially at high pressures and low temperatures. The Van der Waals equation modifies the ideal gas law to account for these factors.
Intermolecular forces determine the strength of attraction between particles. Strong intermolecular forces favour solids, moderate forces favour liquids, and weak forces lead to the gaseous state. These forces influence properties like boiling point, melting point, and compressibility.
Also Read
11 Mar'25 12:08 PM
19 Feb'25 11:49 AM
19 Feb'25 11:23 AM
19 Feb'25 11:14 AM
19 Feb'25 10:31 AM
18 Feb'25 12:15 PM
18 Feb'25 11:59 AM
09 Feb'25 10:16 PM
08 Feb'25 05:31 PM
08 Feb'25 05:28 PM

