Sulphur Dioxide (SO2) - Formula, Sources, Structure, Properties, FAQs
Here in this article we will discuss what is sulphur dioxide, what is nature of sulphur dioxide, what is the formula for sulphur dioxide, colour of sulphur dioxide, sources of sulphur, what sulphur dioxide causes, sulphur ore, where to find sulphur in nature, sulphur dioxide properties and each and every detail of sulphur will be discussed here.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is sulphur dioxide?
- Now we will discuss the formula for dioxide. What is the formula of sulphur dioxide?
- Sulphur Dioxide Properties
What is sulphur dioxide?
SO2 chemical name is sulfur dioxide. Sulphur dioxide when we talk about seems to be solid chemical compound but it is not so. It is a chemical compound but is a gas. We all have some time or other noticed the smell of a burning matchstick, so this burning smell is of the sulphur dioxide. It is not only observed while burning a matchstick but also noticed during natural calamities like volcano eruption and as we know that it is a gas therefore it must be released during some chemical reaction as a by product.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Now we will discuss the formula for dioxide. What is the formula of sulphur dioxide?
When sulphur word is spelt symbol of sulphur comes to one’s mind and the symbol for sulphur is “S” and its atomic number is .now comes the formula for dioxide which is O2. In dioxide molecule two molecules of oxygen are attatched to one another through covalent bond. So as we can observe that while keeping the rules of stoichiometry in mind we can write or sulfur dioxide formula or chemical formula of sulphur dioxide is SO2.
SO2 structure is shown below.
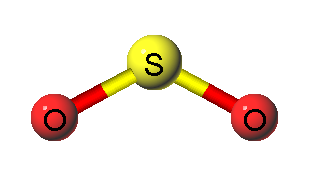
Sources of Sulphur Dioxide
When think about the sources of sulphur different questions arises suppose where to find sulphur in nature? About the sulphur ore and the causes of sulphur dioxide . So we will answer each question one by one. As it is known that the earth’s atmosphere is composed of gases and as there are many gases in the atmosphere so there must be the sulphur dioxide also present. Sulphur dioxide is do found in the atmosphere but in a very small amounts of around 1 ppm but the atmospheres of the other planets do not contain the sulphur dioxide in same amounts.
According to some of the scientific researches it is found that the atmosphere of Venus contains quite a good amount of the sulphur dioxide gas and there the clouds comprises most of this gas in the form of sulphuric acid. Also there are other planets in the universe and each and every planet is supposed to contain the different concentrations of this gas. The other planets which are supposed to contain the higher concentrations of sulphur dioxide are Jupiter and Mars.
We continuously discussing about the sulphur dioxide but before that we should remember that the sulphur dioxide is originated from the sulphur. Therefore we will first discuss the sulphur ore and the sources of sulphur. On earth sulphur is found in abundantly in the form of sulphides and sulphates. It is also obtained during the chemical reactions as a residue when the removal of sulphate products is carried out. Also, ore of sulphur is called.
Sulphur Dioxide Properties
As we all know that there are various properties of each element and here we are talking about the gas that is sulphur dioxide but still it also has two types of properties namely physical properties and the chemical properties. As the name suggests that physical properties are those which are based on their physical appearance like colour, nature, temperature and many more. Chemical properties are those properties that shows the chemical reactions of different substances.
| Related topics link, |
Physical Properties Includes:
The colour of sulphur dioxide is colourless which means it cannot be seen around.
The smell of sulphuric acid is pungent like the nitric acid
It is soluble in water and further when it gets dissolved in water, it forms sulphuric acid.
Its melting point is 201k.
Its boiling point is 263k.
It is acidic in nature.
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 7 The P-block elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 7 The P-block elements
- NCERT notes Class 12 Chemistry Chapter 7 The P-block elements
Chemical Properties
Firstly we will discuss the preparation of the sulphur dioxide
It is simply prepared by burning the piece of sulphur in air in the presence of oxygen and the reaction can be shown below:
![]()
By heating sulphur with concentrated sulphuric acid
![]()
Now we will discuss the reaction between copper and sulphuric acid
![]()
It is also produced when reacted sodium hydrosulphide with dilute hydrochloric acid
![]()
We will now discuss the reaction between sulphur dioxide and potassium dichromate
![]()
Here as this is one of the important reactions discussed so far so it should be noted here that the sulphur dioxide which is actually a gas gets changed into the sulphuric acid through the process of oxidation. Further, it is known that the colour of potassium dichromate is orange so it gets converted or changed into a green colour due to the reduction which took place in the dichromate ions.
Here as it is seen that the sulphur dioxide gets converted into the sulphuric acid this happens because here the process of oxidation took place. Talking on a whole it is a redox reaction because the oxidation and reduction process takes place simultaneously. Now it is interesting to discuss here the one of the compounds of sulphur that is sulphuric diamide. Here as the name suggests that it has the amide group present which makes Sulfamide. It is already known the amide groups are supposed to contain the nitrogen in it.it is further prepared by carrying out the reaction with ammonia.
Here another interesting compound that we hear regularly is magnesium sulphate MgSO2.it is containing the sulphate group that means also a compound of sulphur. It has a great medical importance and we can say this term is often heard in the medication field as it prevents various infections. We have discussed a lot about the sulphur, sulphur products and also each and every thing about the sulphur dioxide.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
The drying agent for sulphur dioxide is concentrated sulphuric acid.
Suppose we take calcium carbonate and hydrochloric acid, then react the two it is observed that the carbon dioxide is released during the reaction.
Liquid sulphur is not much discussed but it forms a ring molecule when proper temperature is provided to it.
It is acidic in nature. It is soluble in water and further when it gets dissolved in water, it forms the sulphuric acid. It further leads acid rain which causes a huge damage to life as well as property. Acid rain is supposed to contain such harmful chemicals only.
Some of the uses of sulphur dioxide are:
It is used to prevent dry fruits from the oxidation or we can also say that acts as a preservative.
It is used for bleaching of various substances as it has a property to get decolourised as it acts as a reductant.
It has a role in our body too but it not completely understood yet.
As we know that in chemistry labs different substances are stored under different conditions in the same way there are certain salts called oxidising salts that are stored in sulphur dioxide.
Also Read
19 Feb'25 11:05 AM
19 Feb'25 11:03 AM
16 Dec'24 11:40 PM
12 Dec'24 05:24 PM
12 Dec'24 12:58 PM
09 Dec'24 11:07 AM
09 Dec'24 11:06 AM
09 Dec'24 10:52 AM
13 Nov'24 07:10 PM