Surface Chemistry - Notes, Topics, Formula, Books, FAQs
Surface chemistry is the branch of chemistry which deals with the study of the type of surface and the species present on it. This anomaly is studied with the help of adsorption and colloidal state which are very useful to understand the chemical and physical characteristics of the substance. The properties of substances are different at the surfaces and in the bulk because the molecules that are present in the bulk are attracted symmetrically in all direction having zero net force but the molecules present at surface familiarize attraction unequally. In this unit, we will study a phenomenon related to the surface.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Important Topics of Surface Chemistry
- Overview of the Chapter Surface Chemistry
- Formulae chart for Surface chemistry:
- How to Prepare for Surface Chemistry?
- Role of Surface chemistry in various chemical processes
- Some important tips to prepare surface chemistry:
- Books prescribed:
Important Topics of Surface Chemistry
Surface Chemistry
Surface chemistry studies phenomena occurring at the interfaces of phases, such as solid-liquid, liquid-gas, or solid-gas. It deals with the processes like adsorption, catalysis, and the behaviour of colloidal particles, which are critical in industries and biological systems.
Difference Between Adsorption and Absorption
Adsorption is the accumulation of molecules on a surface while absorption is the uniform distribution of molecules throughout a substance. Adsorption is a surface phenomenon, whereas absorption occurs throughout the bulk of a material.
Physisorption and Chemisorption
Physisorption involves weak van der Waals forces and occurs at low temperatures, whereas chemisorption involves strong chemical bonds and is specific to the nature of the adsorbate and adsorbent. Chemisorption usually requires activation energy and higher temperatures.
Adsorption Isotherm
An adsorption isotherm depicts the relationship between the amount of adsorbate on the surface and the pressure or concentration of the adsorbate at constant temperature. It helps in understanding adsorption mechanisms.
Freundlich Isotherm
The Freundlich isotherm describes adsorption on heterogeneous surfaces, expressing that adsorption increases with pressure but levels off at high pressures.
Adsorption
Adsorption is the process where molecules adhere to the surface of a solid or liquid. It is widely used in water purification, gas masks, and heterogeneous catalysis. Adsorption depends on factors like temperature, pressure, and surface area.
Catalysis
Catalysis involves substances called catalysts that alter the rate of a chemical reaction without being consumed. Heterogeneous catalysis occurs on the surface of a solid, while homogeneous catalysis occurs in the same phase as the reactants.
Colloids
Colloids are mixtures where particles (1–1000 nm) are dispersed in a medium. Colloids exhibit unique properties like the Tyndall effect and Brownian motion, making them essential in industries like pharmaceuticals, food, and cosmetics.
Preparation and Purification of Colloidal Solutions
Colloidal solutions can be prepared by methods like dispersion (e.g., milling, peptization) or condensation (e.g., chemical reactions). Purification techniques include dialysis, ultrafiltration, and centrifugation to remove impurities.
| Also read, |
Overview of the Chapter Surface Chemistry
It has been known that the surface of a liquid is in a state of unsaturation due to the unbalanced or residual forces which act along the surface of a liquid. Similar to it, the surface of a solid may also have residual forces. Thus, the surface of a solid has a tendency to attract and to retain molecules of gas or liquids with which such surfaces come in contact. This phenomenon of surfaces is termed as adsorption. Differences between Adsorption and Absorption is that Adsorption is a surface phenomenon whereas absorption is a bulk phenomenon in which the substance is symmetrically distributed throughout the body of a solid or liquid to form a solution or a compound.
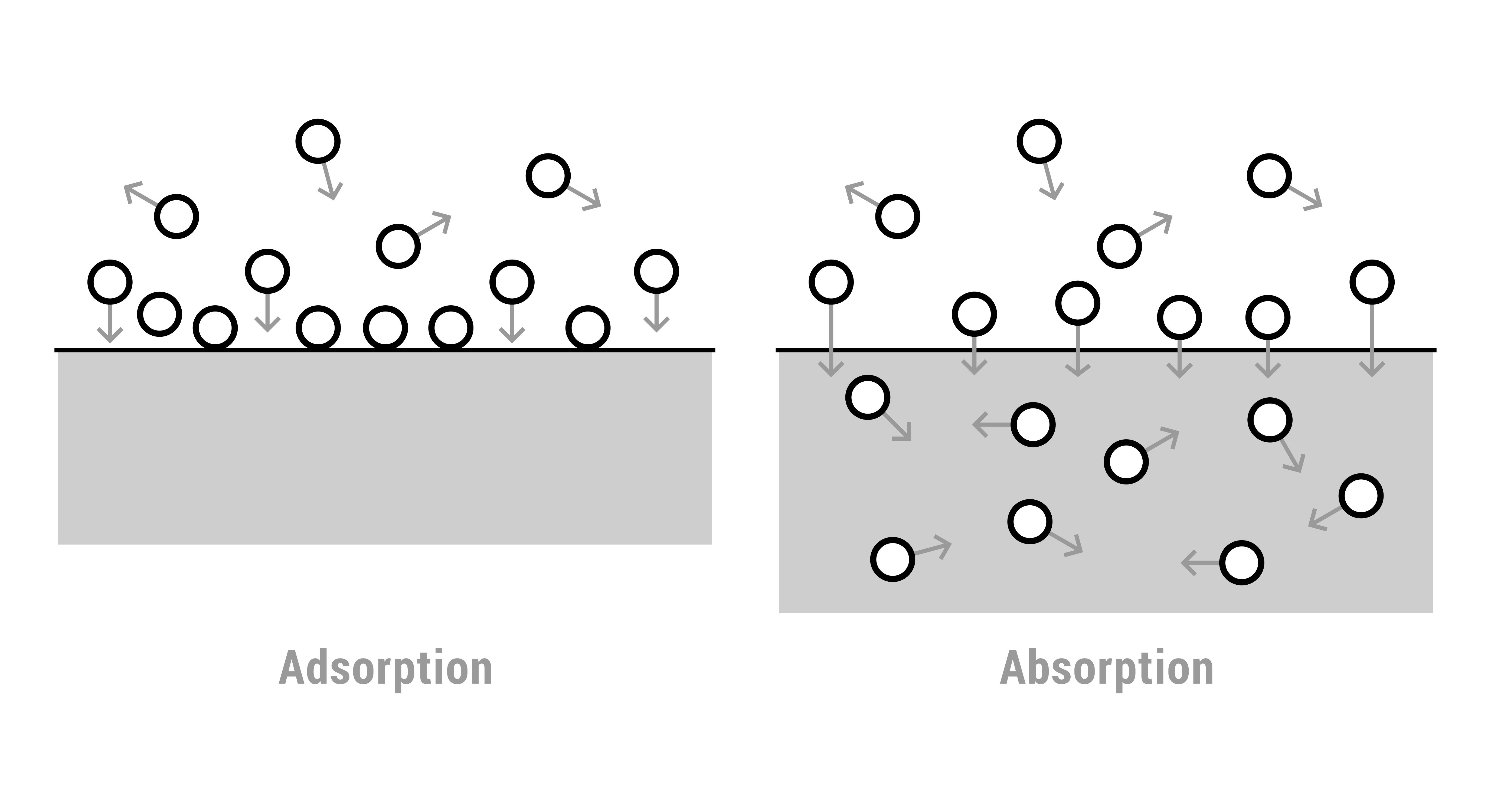
In the case of adsorption, the equilibrium is easily attained in a very short interval of time whereas in absorption the equilibrium takes place slowly.
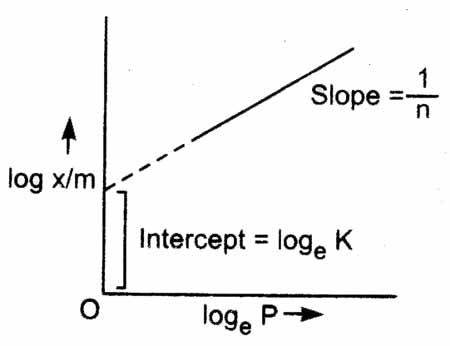
According to Freundlich adsorption isotherm: 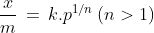
Taking the logarithm of the above equation we get :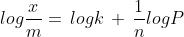
Examples of adsorption and absorption
(i) Water vapour is absorbed by anhydrous calcium chloride while it is adsorbed by silica gel.
(ii) Ammonia is adsorbed by charcoal while it is absorbed by water to form ammonium hydroxide.
NH3+ H2O $\rightarrow$ NH4OH
(iii) Decolourisation of sugar solution by activated charcoal is another example of adsorption. In this example, charcoal adsorbs the colouring material and thus decolourises the solution.
Following them, one will get to know about different types of adsorption, physical adsorption and chemical adsorption.
S.No | Physisorption | Chemisorption |
| 1. | It is due to the van der Waal force of attraction. | The adsorption takes place because of chemical bonds. |
| 2. | It is not specific in nature. | It is specific in nature. |
| 3. | It is reversible. | It is irreversible. |
| 4. | Enthalpy is low. | Enthalpy is high. |
| 5. | Activation energy is not needed. | High activation energy is needed. |
| 6. | Multimolecular layers on adsorbent are formed. | Only unimolecular layers are formed. |
After studying about adsorption the candidate will learn about catalyst. Catalyst is a substance which can change the speed of chemical reaction without undergoing any change in itself whether be it mass or chemical composition at the end of the reaction. This phenomenon is known as catalysis. Catalyst can be broadly divided into two categories:
Homogeneous catalysis: Here reactants and catalyst are in the same phase (liquid or gas)
Heterogeneous catalysis: Here reactants and catalyst are in a different phase.
Talking about the important features of solid catalysts the aspirant will learn about activity and selectivity. By activity of the catalyst, we mean its capacity to increase the speed of chemical reaction. Dependence of activity is on the strength of chemisorption. By selectivity of a catalyst we mean its ability to form particular products excluding others. Moving further in this chapter the candidate will learn about the classification of colloids on the basis of:
1. Size of particles of solute
2. The physical state of the dispersed phase and the dispersion medium
3. Nature of interaction between the dispersed phase and the dispersion medium
4. Types of particles of the dispersed phase
In this part of surface chemistry, the candidate will know about micelle formation, cleansing action of soaps, different methods of preparation of colloids, methods of purification of colloidal solutions and their respective properties which include
Electrophoresis: Electrophoresis is done to confirm the charge on colloid particles. On applying the electric potential across the electrodes, colloid particles move towards one of the electrodes as shown in the figure given below:
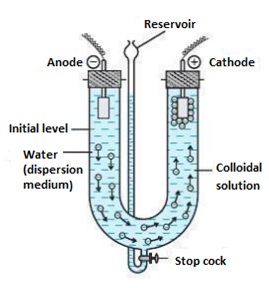
Coagulation or Precipitation(learning about Hardy-Schulze rule): Coagulation is the process when the charge is removed from the colloid particles and these particles come closer to each other and precipitate or coagulate.
Brownian movement: This is the movement of colloidal particles which is observed under a powerful microscope. In this motion, colloidal particles move in zig-zag motion in solution.
Charge on colloidal particles: Colloidal particles carry an electric charge. This charge can be negative or positive but it is always the same for all the particles.
Lastly, one will learn about Emulsions. The emulsion is a colloidal system consisting of immiscible liquids. e.g. milk is an emulsion in which particles of liquid fat are dispersed in water. In common, however, one of the liquids is water and the other, and oily substance insoluble in it. An emulsion is a heterogeneous system consisting of more than one immiscible liquids dispersed in one another in the form of droplets. Such systems possess extremely small stability which is made by the addition of surface-active agents, finely divided solids, etc.
Formulae chart for Surface chemistry:
1. Freundlich adsorption isotherm: 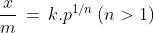
2. Langmuir adsorption isotherm: $\Theta =\, \frac{{K}'P}{1+KP} \, or\, \frac{x}{m}= \, \frac{aP}{1+bP} where\, a ,b\, are \, constant.$
3. $\frac{x}{m}= \, KC^{\frac{1}{n}};\, C= concentration\, of\, solute\, in\, solution$
4. Zeta potential, $Z= \frac{4\pi \eta \mu }{D}$
How to Prepare for Surface Chemistry?
There are some chapters in chemistry which are very hard to gear up with. One of the chapters is surface chemistry. Previously not many questions were asked from this chapter but in recent years since 2012 onwards, direct questions are asked from surface chemistry.
Colloids seem to be the most favorite topic of JEE. So, the aspirant should be thorough with each and every aspect related to colloids like:
Identifying different types of colloids
Methods of preparation of colloids
Electrophoresis
Role of Surface chemistry in various chemical processes
In chemical industries, the principle of catalysis is used for the preparation of chemicals.
Drug selection by doctors like syrup or tablets is based on the reactivity of medicine which depends on surface properties.
In electrical industries, the use in the surface and interface of microchips used in computers.
Protein adsorption on the walls of the intestine.
Adsorption surface phenomena are used for vacuum formation.
Some important tips to prepare surface chemistry:
The candidate must spend ample amount of time in understanding and learning the concepts that too from NCERT.
An aspirant must practice theoretical problems from this chapter
One should practice more than one correct type questions from this chapter.
Various chemical phenomena like electrophoresis must be understood with utmost sincerity.
Books prescribed:
First, you must finish the class XII NCERT textbook and solve each and every example and unsolved question given in it. Then for advanced level preparation like JEE and NEET, you must follow R.C. Mukherjee and P. Bahadur. You must definitely solve the previous year papers. Meanwhile, in the preparation, you must continuously write the mock tests for the depth of knowledge. Our platform will help you to provide with the variety of questions for deeper knowledge with the help of videos, articles and mock tests.
Also read,
Frequently Asked Questions (FAQs)
Adsorption is categorized into physisorption (involving weak van der Waals forces) and chemisorption (involving strong chemical bonds). Physisorption is reversible and occurs at low temperatures, while chemisorption is often irreversible and specific to the adsorbate-adsorbent pair.
For foundational knowledge, NCERT Chemistry (Class XII) is sufficient. For advanced topics, books like Physical Chemistry by P.W. Atkins can be studied.
Colloidal solutions are purified using techniques like dialysis, ultrafiltration, and centrifugation, which remove impurities without affecting the colloidal stability.
Catalysis accelerates chemical reactions by providing an alternative pathway with lower activation energy. Surface chemistry principles explain heterogeneous catalysis, where reactions occur on a solid catalyst’s surface, widely used in industrial processes.
The Tyndall effect is the scattering of light by colloidal particles, making the path of light visible. It is a key property distinguishing colloids from true solutions.
Surface chemistry applications include drug delivery systems, water purification (adsorption of impurities), catalysis in petroleum refining, and designing sensors and nanomaterials for electronics.
Also Read
19 Feb'25 04:59 PM
20 Dec'24 05:25 PM
04 Nov'24 10:45 AM
07 Oct'24 12:46 PM
07 Oct'24 12:44 PM
04 Oct'24 06:04 PM
30 Sep'24 02:35 PM
30 Sep'24 02:28 PM
30 Sep'24 11:36 AM