S-Block Elements - Notes, Overview, Formula, Books, FAQs
The periodic table is divided into 4 blocks, i.e., s-block, p-block, d-block, and f-block. Out of all these blocks, the elements of the s-block form the foundation of chemistry. The s-block elements are divided into two categories, i.e., alkali metals or group 1 elements and alkaline earth metals or group 2 elements. The elements of the s-block have high reactivity and low ionisation energy. These elements are important for both biological and industrial processes. These metals are everywhere around us, from the sodium in the salt we use in our food, to lithium in batteries, to calcium in our bones and teeth. The oxides and hydroxides of s-block elements are alkaline. The general electronic configuration of these elements is ns1 and ns2, respectively.
This Story also Contains
- Important Topics Of S-block Element
- Overview Of The Chapter S-Block Elements
- Diagonal Relationship Between Beryllium and Aluminium
- How To Prepare For S-Block Elements?
- Uses Of S-Block Elements
- Previous Year Question Of S-block Elements
- Prescribed Books
Important Topics Of S-block Element
Group 1 Element- Alkali Metal:
Alkali metals have an electron configuration of ns1, where n is the period number. These elements are well-known for having unique chemical and physical characteristics as well as strong reactivity in the Periodic Table
Anomalous Properties of Lithium:
Lithium, the third element in the periodic table and the lightest metal, is unique among alkali metals. The Anomalous Behaviour Of Lithium is due to the exceptionally small size of its atom and ion and high polarising power.
Alkali Metal Halide:
Alkali Metal Halides are basic compounds made up of alkali metals from Group 1 of the periodic table (such as lithium, sodium, and potassium) and halogens from Group 17 (such as fluorine, chlorine, and bromine).
Some Important Compounds Of Sodium:
- Sodium Chloride And Sodium Hydroxide: Sodium chloride (NaCl), commonly known by the names table salt, rock salt, sea salt, or common salt, is a white, crystalline, hygroscopic solid with a melting point of 1081K and a boiling point of 1713K. Sodium hydroxide (NaOH), or caustic soda, is another crucial chemical with a variety of industrial and laboratory uses. I
- Sodium Carbonate And Sodium Bicarbonate: Sodium bicarbonate, also referred to as baking soda, it is a white, crystalline solid, mostly produced by the Solvay Process. Sodium bicarbonate(NaHCO3)is the result of the reaction between carbon dioxide and a saturated solution of sodium carbonate.
Group 2 Elements: Alkaline Earth Metals
Alkaline Earth Metals are shiny, silvery-white in color, and high density similar to alkali metals. As they are more metallic (i.e. the bonding is stronger), their melting points and boiling points tend to be higher than those of alkali metals. These elements have two electrons in the s-orbital of the valence shell. Their general electronic configuration may be represented as [noble gas]ns2
Anomalous Behaviour of Beryllium:
Berylium differs from the rest of the alkaline earth metals on account of its small atomic size, high electronegativity, and a slight difference in electronic configuration. The different behavior of beryllium is called Anomalous Behaviour Of Beryllium.
Structure Of Beryllium Chloride:
The Structure Of Beryllium Chloride is a polymeric chain structure. Every beryllium atom is tetrahedrally linked to four chlorine atoms, while every chlorine atom connects two beryllium atoms.
Some Important Compounds Of Calcium:
- Calcium Oxide (quick Lime) And Calcium Hydroxide (slaked Lime): Calcium oxide is a white, caustic, alkaline, crystalline solid at room temperature. It is prepared by the thermal decomposition of calcium carbonate (limestone). Calcium Hydroxide is also known as slaked lime. It is prepared by the reaction of lime with water.
- Calcium Carbonate And Calcium Sulphate(plaster Of Paris): Calcium carbonate, CaCO₃ is obtained in most rocks, primarily as the minerals calcite and aragonite. It forms the principal constituents of pearls, shells of marine organisms, and eggshells. Calcium sulphate, CaSO₄ occurs in a number of hydrated forms; the most well-known are gypsum, CaSO₄·2H₂O, and plaster of Paris, CaSO₄·0.5H₂O.
Diagonal Relationship Between Beryllium And Aluminium:
Several aspects of beryllium and aluminum are the same, such as being amphoteric, the formation of covalent compounds, and resistance to acid attacks. Also, beryllium and aluminum combine to form some covalent compounds with certain elements. This similarity between these two is called the Diagonal Relationship Between Beryllium And Aluminium.
Also Read,
- Preparation Properties and Uses Baking Soda
- Baking Soda Washing Soda Plaster of Paris
- Caustic Soda Preparation Properties and Uses
- Na2CO3
- Sodium Sulfate
- Potassium Hydroxide
- Sodium Oxide
Overview Of The Chapter S-Block Elements
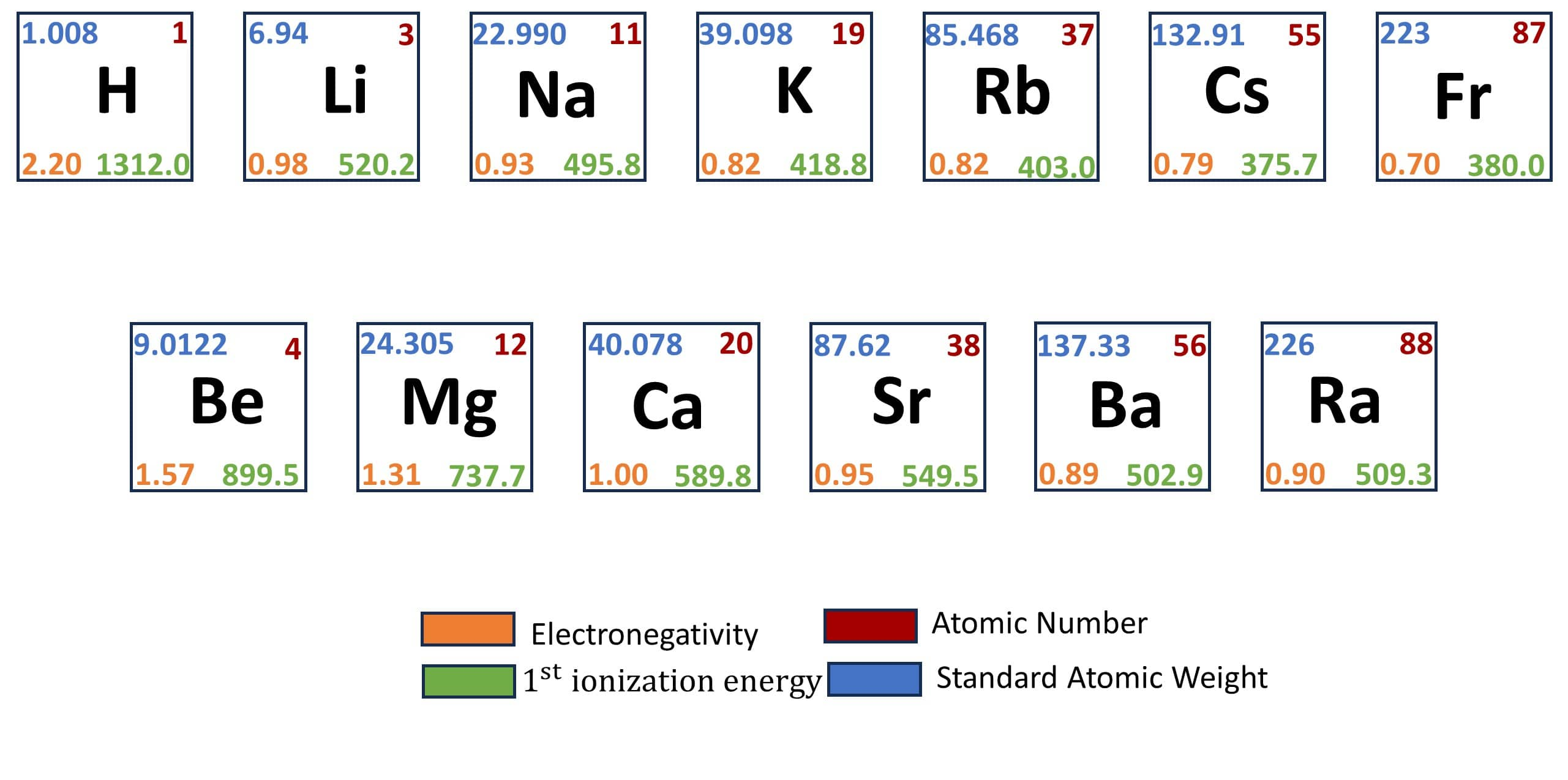
The s-block elements are those in which the outermost electrons exist in the s-orbital. Alkali and alkaline earth metals are respectively the members of group 1 and group 2 elements. In this chapter, there are various important properties that you need to learn, such as electronic configuration, ionization enthalpy, hydration enthalpy, chemical properties, etc.
Group 1 Elements: Alkali Metals
(i) Electronic Configuration: The alkali metals or group 1 elements have the valence shell electronic configuration of ns1. The table given below describes the electronic configuration of the alkali metals.
| Element | Symbol | Electronic configuration |
| Lithium | Li | [He] 2s1 |
| Sodium | Na | [Ne] 3s1 |
| Potassium | K | [Ar] 4s1 |
| Rubidium | Rb | [Kr] 5s1 |
| Caesium | Cs | [Xe] 6s1 |
| Francium | Fr | [Rn] 7s1 |
(ii) Atomic and Ionic Radii: Alkali metal atoms have the largest sizes in their respective periods. This atomic size decreases as we move along the period and increases as we move down the group.
(iii) Ionization Enthalpy: The ionization enthalpy of the alkali metal atoms is low as compared to other atoms in their respective periods. This is because of the larger size of these atoms. As we move down the group, the ionization enthalpy further decreases because of the larger size of the atoms.
(iv) Chemical Properties
- Reactivity towards air: Alkali metals react too fast with oxygen and form oxides. Lithium, being the smallest element, forms monoxide, sodium forms peroxide, and other large metals form superoxides.
$$\begin{aligned} & 4 \mathrm{Li}+\mathrm{O}_2 \rightarrow 2 \mathrm{Li}_2 \mathrm{O} \\ & 2 \mathrm{Na}+\mathrm{O}_2 \rightarrow \mathrm{Na}_2 \mathrm{O}_2 \\ & \mathrm{~K}+\mathrm{O}_2 \rightarrow \mathrm{KO}_2\end{aligned}$$
- All these metals in their oxides have the oxidation number equal to +1.
- Reactivity towards water: Alkali metals react with water to form hydroxides and dihydrogen.
$$2 \mathrm{Na}+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2 \mathrm{NaOH}+\mathrm{H}_2$$
- Reactivity towards dihydrogen: Alkali metals react with dihydrogen and form hydrides.
$$2 \mathrm{Na}+\mathrm{H}_2 \rightarrow 2 \mathrm{NaH}$$
- Reactivity towards halogens: Alkali metals react too fast with halogen elements to form alkali halides.
$$2 \mathrm{Na}+\mathrm{Cl}_2 \rightarrow 2 \mathrm{NaCl}$$
- Alkali metals with ammonia: These metals dissolve in liquid ammonia giving the deep blue solution.
$$\mathrm{M}+(\mathrm{x}+\mathrm{y}) \mathrm{NH}_3 \rightarrow\left[\mathrm{M}^2\left(\mathrm{NH}_3\right) \mathrm{x}\right]^{+}+\left[\mathrm{e}\left(\mathrm{NH}_3\right) \mathrm{y}\right]^{-}$$
Anomalous Properties Of Lithium
The deviation of the behavior of lithium from its respective elements is because of two factors:
(i) The exceptionally small size of the atom
(ii) High polarising power
Because of these two factors, lithium has some points of difference from other alkali metals and some similarities with magnesium.
(i) Differences Between Lithium And Other Alkali Metals
- Lithium is harder, and its melting point and boiling point are also higher than other alkali metals.
- Lithium is less reactive than other alkali metals.
- Lithium does not form solid hydrogen carbonate, unlike other alkali metals.
- Lithium does not form ethynide on reaction with ethyne, unlike other alkali metals.
- Lithium nitrate on heating gives lithium oxide but other alkali metal nitrates produce metal nitrite.
- Fluorides and oxides of lithium are less soluble in water than those of other alkali metals.
(ii) Similarities Between Lithium And Magnesium
The similarity between lithium and magnesium is also known as the diagonal relationship with magnesium. This similarity between lithium and magnesium arises because of their similar sizes.
- Lithium and Magnesium, both are harder and lighter than their respective group elements.
- Their oxides and hydroxides are less soluble.
- Both oxides of lithium and magnesium do not combine with excess oxygen.
- The chlorides of both lithium and magnesium are soluble in ethanol.
- Both form nitride in reaction to nitrogen, known as lithium nitride and magnesium nitride.
Some Important Compounds Of Sodium
There are some important compounds of sodium from an industrial basis. Their production and uses are discussed below.
(i) Sodium Carbonate
(ii) Sodium Chloride
(iii) Sodium Hydroxide
(iv) Sodium hydrogen carbonate
Group 2 Elements: Alkaline Earth Metals
(i) Electronic Configuration: The alkaline earth metals or group 2 elements have the valence shell electronic configuration of ns2. The table given below describes the electronic configuration of the alkali metals.
| Element | Symbol | Electronic configuration |
| Beryllium | Be | [He] 2s2 |
| Magnesium | Mg | [Ne] 3s2 |
| Calcium | Ca | [Ar] 4s2 |
| Strontium | Sr | [Kr] 5s2 |
| Barium | Ba | [Xe] 6s2 |
| Radium | Ra | [Rn] 7s2 |
(ii) Atomic and Ionic Radii: Alkaline earth metal atoms have larger sizes than other metal atoms in their respective periods but are smaller than the alkali metals. This atomic size decreases as we move along the period and increases as we move down the group.
(iii) Ionization Enthalpy: The ionization enthalpy of the alkali metal atoms is low as compared to other atoms in their respective periods but higher than the alkali metal atoms. As we move down the group, the ionization enthalpy further decreases because of the larger size of the atoms.
(iv) Chemical Properties
- Reactivity towards air and water: Beryllium and magnesium are usually inert as they are covered with an oxide film on their surface. But the powdered beryllium burns in air and forms beryllium oxide. Other heavier elements of this group readily react with air and form oxides.
- Reactivity towards halogens: All alkaline earth metals combine with halogen to form halides.
$$\mathrm{Mg}+\mathrm{Cl}_2 \rightarrow \mathrm{MgCl}_2$$
- Reactivity towards hydrogen: Except beryllium, all other alkaline earth metals combine with hydrogen to form their hydrides.
- Reactivity towards acids: The alkaline earth metals react with acids and liberate hydrogen.
$$\mathrm{Mg}+2 \mathrm{HCl} \rightarrow \mathrm{MgCl}_2+\mathrm{H}_2$$
- Alkaline earth metals with ammonia: These metals dissolve in liquid ammonia and give a blue-black solution.
$$\mathrm{M}+(\mathrm{x}+\mathrm{y}) \mathrm{NH}_3 \rightarrow\left[\mathrm{M}\left(\mathrm{NH}_3\right) \mathrm{x}\right]^{2+}+2\left[\mathrm{e}\left(\mathrm{~N}_3\right) \mathrm{y}\right]^{-}$$
Diagonal Relationship Between Beryllium and Aluminium
-
Aluminum and Beryllium both have oxide film on their surface and thus are not easily attacked by acids.
-
Both aluminum hydroxide and beryllium hydroxide react with alkali to form beryllate and aluminate ions
- Both aluminum and beryllium react with chlorine and form polymeric chlorides. These chlorides are soluble in organic solvents.
- Aluminum and Beryllium ions, both have a strong tendency to form complexes.
Some Important Compounds Of Calcium
(i) Calcium Oxide or Quick Lime(CaO)
(ii) Calcium Hydroxide (Slaked lime), Ca(OH)2
(iii) Calcium Carbonate, CaCO3
(iv) Calcium Sulphate (Plaster of Paris), CaSO4·H2O
How To Prepare For S-Block Elements?
-
This chapter is a part of inorganic chemistry. It is completely theory-based and very easy to learn, no need to memorize any formula.
-
Before reading this chapter, first, you must have the basic knowledge of the chapter - periodic classification of elements.
-
You must also learn why there are some elements like Boron and Carbon show anomalous behavior with respect to other elements in their group.
-
Rest this complete chapter is very simple, just be regular and be consistent in your numerical practice.
Uses Of S-Block Elements
S-block elements have a large number of uses in our daily lives s-block elements form various compounds or salts that are helpful in our daily lives.
- They are used for the manufacture of various paper, soap, artificial silk, and glass.
- s-block elements are used in petroleum refining and for mercerizing cotton
- Alkaline earth metals are used in the production of medicines such as antacids and medicines used in cancer treatments.
Previous Year Question Of S-block Elements
Question:1 Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric field?
a) Li
b) Na
c) K
d) Rb
Solution:
Answer is option (a). The ionic size of Li+ is lowest and the surface charge density is highest among all other alkali metals. Therefore, Li+ is most heavily hydrated among all alkali metal ions. Effective size of Li+ ion aqueous solution is therefore the largest. Due to its large size, its ionic mobility is lowest under electric field.
Question 2: Which alkali metal has the least ionization enthalpy?
a) Na
b) K
c) Rb
d) Cs
Solution:The
The answer is option (d). Ionisation enthalpy of the alkali metals are considerably low and it decreases as we move down the group from Li to Cs. The effect of increasing number of shells down the group outweighs the increasing nuclear charge and thus the outermost electron is very loosely bounded to the nucleus. Hence, lesser energy is required to remove the electron from its outermost shell. Therefore, Cs has the least ionisation enthalpy.
Question 3: Which of the following are the correct reasons for anomalous behaviour of lithium?
(i) Exceptionally small size of its atom
(ii) Its high polarising power
(iii) It has high degree of hydration
(iv) Exceptionally low ionisation enthalpy
1) (i) and (ii)
2) (iii) and (iv)
3) (ii) and (iii)
4) None of above
Solution:
Anomalous Behaviour of Lithium: The anomalous behavior of lithium is due to the
- Exceptionally small size of its atom and ion
- High polarising power.
As a result, there is an increased covalent character of lithium compounds, which is responsible for their solubility in organic solvents. Further, lithium shows a diagonal relationship to magnesium, which has been discussed subsequently. Lithium is much harder. Its m.p. and b.p. are higher than the other alkali metals. Lithium is the least reactive but the strongest reducing agent among all the alkali metals.
The answer is the option (i) and (ii) Li has an exceedingly small size and high nuclear charge due to which it shows high polarizing power.
Hence, the answer is the option (1).
Practice more questions from the link given below
For more questions to practice, the following MCQs will help in the preparation of competitive examinations
Prescribed Books
First, you must finish the class XI textbook and solve each and every example and unsolved question given in it. Then, for advanced level preparation like JEE and NEET, you must follow O.P. Tandon or Solomons and Fryhle. You must definitely solve the previous year's papers.
Frequently Asked Questions (FAQs)
- Alkali Metals: They are called "alkali" because their oxides and hydroxides are soluble in water and form alkaline solutions (bases).
- Alkaline Earth Metals: They are called "alkaline earth" because their oxides and hydroxides are infusible at high temperatures (hence "earth") and also form alkaline solutions.
The two main groups of S-block elements are:
- Group 1: Alkali Metals (Lithium, Sodium, Potassium, Rubidium, Cesium, Francium)
- Group 2: Alkaline Earth Metals (Beryllium, Magnesium, Calcium, Strontium, Barium, Radium)
Alkali metals react with water to form hydroxides and hydrogen gas. They react vigorously with water.
Example: 2Na(s)+2H2O(l)→2NaOH(aq)+H2(g)
S-block elements are those elements that are found in group 1 and group 2 of the periodic table. The elements of Group 1 are called alkali metals. Some of these metals are lithium, sodium, and potassium. While the elements of group 2 are called alkaline earth metals, some of these metals are beryllium, magnesium, etc.
There are various applications of s-block elements. Sodium is used as a food preservative, calcium is essential for bone health, and calcium is also used in cement and plaster. Potassium is used in fertilizers, magnesium is used in automobiles and aerospace.
Common compounds of s-block elements are:
- Sodium chloride (NaCl)
- Calcium carbonate (CaCO3)
- Magnesium oxide (MgO)
Some common properties of s-block elements are:
- High reactivity
- Low melting and Boiling point
- Low density
- Good conductors of heat and electricity
- Basic oxides and hydroxides
