The Story of Washing Soda (Na2CO3) - Solvay Process, Properties, Structure, Uses, FAQs
The Story of Washing Soda Na2CO3.10H2O
Sodium carbonate is the chemical name of washing soda is a white, odorless powder mainly used for washing purposes. Washing soda has many applications from household uses to industrial applications. It is basically an alkaline compound with a high basic character and has the ability to remove dirt from the clothes during washing. The chemical formula of washing soda is Na2CO3.10H2O. Washing soda is a hygroscopic compound that can absorb water from the moisture present in the air.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- The Story of Washing Soda Na2CO3.10H2O
- What is Solvay Process?
- Properties of washing soda
- Uses of washing soda
- Structure of Sodium Carbonate
It also has the name soda ash since sometimes it is made from the ashes of plants and also it is made by placing baking soda in the oven. And it is the hydrated salt of the chemical compound sodium carbonate. The alkalinity of washing soda can be tested using litmus paper, as the solution of washing soda turns the red litmus to blue. Washing soda contains 10 molecules of water if this water is not present it is given the name sodium carbonate anhydrate. The formula for which is Na2CO3. The sodium carbonate decahydrate formula is Na2CO3.10H2O. Na2CO3.10H2Ochemical name is sodium carbonate decahydrate.

Figure showing image of washing soda
Due to the presence of 10 water molecules washing soda has also another name that is sodium carbonate decahydrate. The elements present in washing soda are two sodium and one carbonate ion and is an ionic compound. Because the bond present in them is an ionic bond. The molar mass of washing soda is 106 grams per mole with a density of 2.54 grams per centimeter cube. as the compound is alkaline the taste of washing soda is an alkaline taste with a greyish white color. The melting point of washing soda is 851 degrees Celsius and the boiling point of 1600 degrees Celsius. The compound is a stable compound only at ordinary temperature if the temperature is high the compound dries out that is the water molecules present in them got dehydrated.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is Solvay Process?
Solvay process is a process that is used for manufacturing washing soda. It is a huge process as it requires four steps to manufacture the washing soda. The process is simple but it includes many steps. And care must be given in order to be free from contamination.
Things required for the production of washing soda
1. Sodium chloride
2. Limestone
3. Ammonia gas
The four steps involved in the manufacture of washing soda are described below.
1. Brine purification
2. Sodium hydrogen carbonate formation
3. Sodium carbonate formation
4. Ammonia recovery
Step1: Brine purification
The first step is the production of brine, which is the saturated solution of sodium chloride. The obtained brine is then concentrated by the evaporation process and the impurities present in them are removed by precipitation. The purification of brine is very important as it is very important to prevent the presence of impurities in the final product that is washing soda. When the impurities are removed the brine solution is filtered and allowed to pass the ammonia tower for dissolving the ammonia. When the purification is completed the brine is then allowed into contact with the ammonia gas.
Step2: Sodium hydrogen carbonate formation
The brine is now ammoniated and hydrogen carbonate is precipitated by slowing the brain into contact with carbon dioxide. The following is the reaction taking place for the formation of sodium hydrogen carbonate.
NH3(aq)+CO2(g)+NaCl(aq)+H2O→NaHCO3(s)+NH4Cl(aq)
Step3: Sodium carbonate formation
The obtained sodium hydrogen carbonate is treated at 300 degrees celsius to produce the sodium carbonate. The following equation shows the reaction taking place in the formation of sodium carbonate.
2NaHCO3→Na2CO3+CO2+H2O
Carbon dioxide is formed as a by-product and it then recycles the back for the formation of sodium hydrogen carbonate. Now the obtained sodium carbonate undergoing recrystallization to form the washing soda is also called a basic salt.
Step4: Ammonia recovery
By treating calcium hydroxide with ammonium chloride the ammonia can be easily recovered. The obtained ammonia can be recycled back for the Solvay process. And calcium chloride is obtained as a byproduct. The following equation shows the recovery of ammonia.
NH4Cl+Ca(OH)2→2NH3+CaCl2+H2O
| Related topics link, |
Properties of washing soda
Washing soda is a white crystalline solid mainly existing in the decahydrate form. It is basic in character. In the presence of heat losses water molecules and form an anhydrate compound called sodium carbonate which also has industrial applications. The reaction taking place is
Na2CO3.10H2O → Na2CO3+10H2O
Washing soda is soluble in water and is alkaline in nature that it turns red litmus to blue. Washing soda and baking soda are different although they sound similar. Baking soda is sodium bicarbonate and it can be used for removing laundry odor. Washing soda is used for cleaning clothes. Washing soda removes dirt and grease from the clothes by converting the dirt and greasy components into water-soluble products that can be easily then washed away upon rinsing with water.
Also, students can refer,
- NCERT solutions for Class 12 Chemistry Chapter 10 The S-Block Elements
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 10 The S-Block Elements
- NCERT notes Class 12 Chemistry Chapter 10 The S-Block Elements
Uses of washing soda
- Washing soda is used for cleaning purposes in the household and also for industrial uses.
- It has much application in many industrial fields like paper, textile, soap, detergent, etc.
- It can be used for softening hard water, both temporary and permanent hardness can be removed.
- For the manufacture of glass washing soda is one of the important components.
- For the laundry purpose washing soda is one of the important agents.
- For the manufacture of borax washing soda is used.
- For the deep cleaning of washing machines washing soda is used.
- Washing soda together with boiling water can be used for unclogging drains.
Structure of Sodium Carbonate
Sodium carbonate contains two sodium cations with a charge of +1, and it is surrounded above the anion carbonate. The bond present on them is ionic. The below figure shows the structure of sodium carbonate.
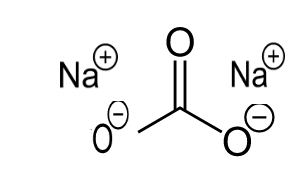
Figure Structure of sodium carbonate
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Na2CO3.10H2O
Soda ash. Since it is prepared from the ashes of plants and also by the burning of baking soda by placing them on the oven washing soda got another name as soda ash.
Washing soda, Na2CO3.10H2O is an inorganic compound with many industrial and household uses. It also has the name soda ash since it was prepared from the ashes of plants and by burning baking soda in an oven. Washing soda as the name itself suggests it has a very important use in washing purposes. When washing soda comes in contact with dirt and greasy components it will convert these water-insoluble substances into water-soluble components and can be easily removed by rinsing with water. Thereby made the cleaning process easy. Washing soda is prepared in large quantities by the process named Solvay.
Washing soda is a base, the reason is when washing soda is dissolved in water the compound dissociates into its respective ions as sodium hydroxide and the weak acid, carbonic acid. And also the solution turns the red litmus to blue which is the indication of basic character.
Sodium carbonate.
11.6 is the pH of washing soda and is a base.
Sodium carbonate decahydrate.
Washing soda is prepared by the process named Solvay process. It is a process involving four steps but it is a simple process. Brine purification, Sodium hydrogen carbonate formation, Sodium carbonate formation, and Ammonia recovery are the four steps involved in the Solvay process.
Greyish white color.
Also Read
16 Jun'25 11:41 PM
19 Feb'25 10:44 AM
17 Dec'24 10:24 AM
17 Dec'24 10:21 AM
29 Nov'24 12:13 PM
30 Sep'24 02:31 PM
30 Sep'24 11:46 AM
26 Sep'24 06:10 PM

