Tyndall Effect - Definition, Examples, FAQs
What is Tyndall Effect?
Tyndall effect definition: The Tyndall effect meaning is a effect of Tyndall is that the particles on the particle emit rays of light directed toward them. This effect of Tyndall effect dispersion of light is demonstrated by all colloidal solutions and other excellent suspensions. The Tyndall effect is shown by colloidal solution
JEE Main 2025: Chemistry Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
Therefore, it can be used to ensure that the given solution is a column. The intensity of diffused light depends on the density of the colloidal particles and the density of the incandescent light. When a ray of light passes through a colloid, the colloidal particles present in the solution do not allow this beam to pass completely. Light collides with colloidal particles and is dispersed (deviates from its normal line, which is a straight line).
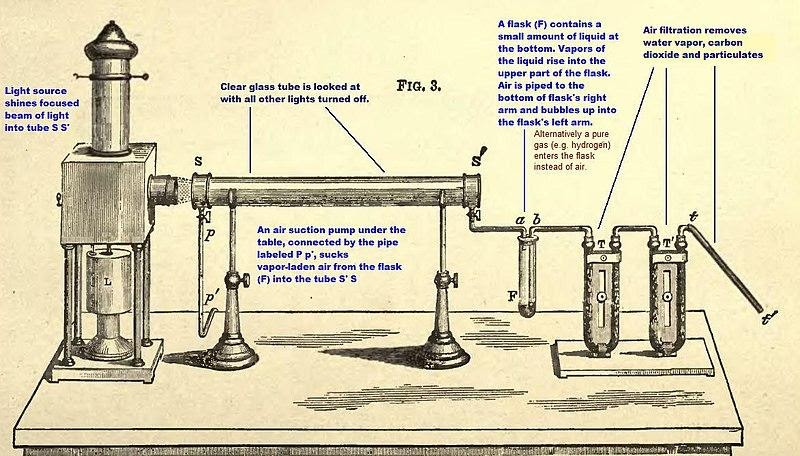
The above figure shows the Tyndall effect diagram.
In general, blue light is dispersed on a larger scale compared to a red light. This is because the length of the blue light is less than that of the red. This is the reason why the smoke from motorcycles sometimes looks blue. The particle diameter that causes the effect of Tyndall can range from 40 to 900 nanometers (1 nanometre =10-9 meters). In comparison, the average light intensity ranges from 400 to 750 nanometers.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Tyndall Effect Example
Milk is a colloid that contains fat and protein globules. When a ray of light is directed to a glass of milk, the light disperses. This is a good example of Tyndall's effect.
When the light is turned on in a foggy place, a path of light is visible. In this case, water droplets in the mist are subject to light scattering.
Opalescent glass has a blue appearance when viewed from the side. However, orange light comes out when the light shines through a glass.
How does the effect of Tyndall look with the Blue Eye colour: The main difference between irises blue, brown, and black is the amount of melanin in one of its layers. The layer in the blue iris has very low levels of melanin in it compared to the black iris, which makes it more durable. When light is an event in this changing layer, it is scattered because of Tyndall.
Since the blue light has a shorter proportion of light compared to the red light, it spreads on a larger scale. Another deep layer of iris absorbs diffused light. Since most of the scattered light is blue, these irises acquire their blue colour.
Several cases involve diffusion of light. The disintegration of Rayleigh and Mie are examples of such situations. The clear sky is blue due to the diffusion of light by air particles, which is an example of Rayleigh's dispersion. However, when the sky is clouded over, the clouds are relatively large and tend to scatter light, which is an example of Mie scattering.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Two conditions you should be satisfied to see the effect of Tyndall: The diameter of the dispersed particles should be less than the length of light used. The return indicators for the distribution rate and the dispersed phase should differ by the size of the maximum scale.
The Tyndall effect is due to scattering of light in the colloidal scattering, while the lack of light is a real solution. This result is used to determine whether the mixture is a real solution or a colloid.
It is caused by the light of the incident radiation from the particles, from the inner walls of the particles, and the retraction and separation of the radiation as it passes through the particles. Some eponyms include Tyndall beam (light dispersed by colloidal particles).
Distribute light through water droplets in the air. Lighting a flashlight in a milk glass. One of the most intriguing examples of Tyndall effect is the iris in blue. The flexible layer over the metal that causes the spread of blue light makes the eyes look blue.
The effect of Tyndall is the effect of light scattering on colloidal scattering while not showing scattering in real solution. This result is used to determine whether the mixture is a real solution or a colloid.
The Tyndall effect is used in commercial and laboratory settings to determine the particle size of aerosols. Opalescent glass shows the effect of Tyndall. The glass looks blue, but the bright light on it looks orange. The colour of the blue eyes comes from Tyndall, which spreads through the flexible layer above the eyeball.
Water and milk form a colloidal solution that reflects the effect of Tyndall because the larger particles that cause light scattering and reflect the effect of Tyndall.
Since colloids have particles in them that transmit the transmitted light, they reflect Tyndall's influence. The sugar solution is a real solution and not a colloid solution. Therefore, the effect of Tyndall is not indicated by the sugar solution.
The aqueous solution below the critical micelle concentration is not a colloid solution and the soapy water solution above the critical micelle concentration is a colloidal solution. Therefore, the effect of Tyndall effect dispersion of light l will be reflected in the gift solution over the delicate micelle concentration.
Tyndall's effect was first discovered (and named) by Irish naturalist John Tyndall.
The effect of Tyndall is that the particles on the particle emit rays of light directed toward them. This effect of Tyndall effect dispersion of light is demonstrated by all colloidal solutions and other excellent suspensions.
Also Read
18 Nov'22 04:57 PM
21 Jul'22 03:24 PM
11 Jul'22 05:39 PM