Types of Chemical Reactions - Explanation, Examples, FAQs
What is a Chemical Reaction?
A chemical reaction occurs when the bonds between reactant molecules are broken and new bonds are established between product molecules, resulting in the formation of a new substance. The reactants are the substances that react during a chemical reaction, whereas the products are the chemicals that are created during a chemical reaction. Chemical reactions may be found everywhere around us, from our bodies' food metabolism to how the light we receive from the sun is produced through chemical reactions. It is crucial to understand physical and chemical changes before starting with chemical reactions.
The best example of physical and chemical change is a burning candle. Take a candle and put it on the table. We can see how the candle turns to wax as time goes on. The candle will go out if you cover it with a jar. The burning of the candle is a chemical change, whereas the conversion of the candle to wax is a physical change in the demonstration. A physical change primarily results in a change in the state of the substance, whereas a chemical change primarily results in the formation of a new substance in which energy is either released or absorbed. As a result, we can deduce that chemical changes are followed by physical modifications.
Types of Chemical Reactions with Example
Chemical reactions are classified into several categories based on a variety of characteristics.The list of various types of chemical reactions are:
Decomposition Reaction
Combination Reaction
Combustion Reaction
Neutralisation Reaction
Single displacement Reaction
Double Displacement Reaction
Precipitation Reaction
Redox Reaction
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Decomposition Reaction
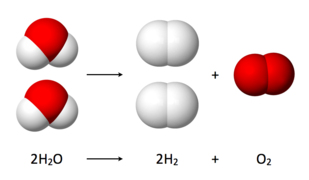
Molecules or compounds decompose into two or more simpler chemically new entities in a decomposition reaction. Take, for example, water electrolysis. Water breaks down into hydrogen and oxygen during electrolysis, which have completely different properties than water.
Combination Reaction
Combination reaction definition: Two or more molecules are chemically joined to generate a new substance (compound) in a combination reaction. The reactions of combination and decomposition are completely contradictory.
Example of combination Reaction : When we burn magnesium ribbon (or magnesium), we get grey – black ash of magnesium oxide.
2 Mg + O2 → 2 MgO
Types of Combination Reaction
1. An element combines with another element to generate a new compound in the first type of combination reaction.
Example: When nitrogen and hydrogen are run over finely split iron, they react to form ammonia, a pungent-smelling gas.
N2 + 3 H2 → 2 NH3
2. An element combines with a compound in the second types of reaction.
When a chemical SO2 reacts with oxygen to form sulphur trioxide as an example.
2SO2 + O2 → 2SO3
3. Two or more compounds mix to generate a new compound in the third type of combination.
Example: Slaked lime is a reaction in which lime reacts vigorously with water to produce a white powder of Calcium hydroxide.
CaO + H2O → Ca(OH)2
Combustion Reaction
It is a type of exothermic reaction that releases energy in the form of heat. It is a reaction that occurs when fuel reacts with an oxidant (usually air oxygen) to generate smoke, water, and heat. When we burn methane, for example, we get carbon dioxide and water.
CH4 + 2 O2 → CO2 + 2 H2O
Neutralization Reaction
Acid and base react with each other to produce salt and water in the neutralisation reaction. Hydrochloric acid, for example, interacts with sodium hydroxide (base) to produce sodium chloride (salt) and water.
HCl + NaOH → NaCl + H2O
Related Topics link, |
Single Displacement Reaction
In these reactions, more reactive metal displaces less reactive metal from its salt.Reactivity series can be used to determine the products in these reactions. The reactivity series is a set of items grouped in decreasing order of reactivity. It indicates that the elements at the top of the reactivity scale are more reactive than those at the bottom. A single displacement reaction is the reaction of potassium with magnesium chloride. Because potassium is more reactive than magnesium, it displaces magnesium from its salt in this reaction. Potassium is the most reactive element and is found at the top of the reactivity scale.
2K + MgCl2 → 2 KCl + Mg
Double Displacement Reaction
Two aqueous ionic compounds exchange their ions (mainly cations) and form two new compounds in these reactions. Potassium nitrate, for example, interacts with aluminium chloride to produce aluminium nitrate and potassium chloride.
KNO3 + AlCl3 → KCl + Al (NO3) 3
Precipitation Reaction
These reactions result in the formation of an insoluble precipitate. Two soluble salts in aqueous solutions are joined in precipitation processes to generate an insoluble precipitate.
AgNO3 (aq) + KCl (aq) → AgCl + KNO3 (aq)
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 1 Some basic concepts of Chemistry
- NCERT notes Class 11 Chemistry Chapter 1 Some basic concepts of Chemistry
Redox Reaction
Redox reactions are chemical reactions that involve both oxidation and reduction at the same time. The addition of oxygen is oxidation, while the addition of hydrogen is reduction (or removal of oxygen). A redox reaction is the reaction of copper oxide with hydrogen. In this reaction, hydrogen is oxidised by gaining one oxygen atom, whilst copper oxide is reduced by eliminating oxygen.
CuO + H2 → Cu + H2O
Types of Chemicals Reactions | General Reaction or Types of Chemical Equations: |
| AB → A + B |
| A + B → AB |
| Acid + base → Salt + Water |
| A + O2 → H2O + CO2 |
| A + BC → AC + B |
| A + B (soluble salt) → precipitate + C (soluble salt) |
Difference Between Combination and Decomposition Reaction
Combination Reaction | Decomposition Reaction |
Combination reactions occur when several reactants combine to produce a single product | Decomposition reactions occur when one reactant breaks down into numerous products. |
2 Mg + O2 → 2 MgO | 2H2O → 2H2 + O2 |
Synthesis Reaction
When two or more reactants combine to generate a single product, it is called a synthesis reaction. A + B → AB is a general equation that describes this type of reaction. The reaction of sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl) is an example of a synthesis reaction.
Na + Cl → NaCl
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: